Venous endoscopy
Stelian Stefanita MOGOANTA
Craiova, The IInd General Surgery
Department, Craiova, Romania
ABSTRACT
Venous endoscopy, one of the latest technologies of invasive exploration of the veins, holds unexpected therapeutic potential. It allows the visualization of normal and pathological endovenous structures. While most pathological structural changes are usually acquired, some of them (intraluminal cords, strips) may be remnants of vein genesis. Normal valve dynamics vary with the pressure of the flow and so reflect functional and structural asymmetry, a fundamental characteristic of the living world. Information provided by venous endoscopy has allowed us to establish a rigorous classification of valvular lesions, but also to describe other types of endovenous lesions (endophlebitis, proliferative endophlebitis, intimal sliding, transvalvular thrombus, etc). Endoscopic and ultrasonographic study of reflux has helped clarify the pathogenesis of functional lesions of the cusps (commissural reflux slit, commissural reflux channel) and the consequences of eccentric-onset reflux (eccentric varicose dilation under the cusps). Venous hemodynamics is a field influenced by many traditional non-selective acquisitions and extrapolations of classical Newtonian fluid mechanics, far away from the reality of functional venous circulation, a living piping system in which flows a living anisotropic fluid.
The use of adapted and improved tools and video-endoscopic systems may allow endoscopic valvuloplasty and minimally invasive embolectomy avoiding valve damage, and help with stent placement etc, thereby resulting in better and faster recovery for patients with chronic venous disease.
Venous endoscopy is one of the most recent exploration technologies to have made possible the direct visualization of normal and pathological endovenous structures. The information acquired has shed some light on venous hemodynamics is a field influenced by many traditional non-selective acquisitions and extrapolations of classical Newtonian fluid mechanics, far away from the reality of functional venous circulation, a living piping system in which flows a living anisotropic fluid. The anisotropy of blood makes it impossible to use a laminar flow model, especially in the venous circulation, where deformation and changes in flow occur continuously. In addition, the presence of valves acting as true diaphragms and the frequent breaks in venous flow, even in physiological conditions, show the extraordinary complexity and variability of venous hemodynamics. Venous modeling is related to the temporal perishability of living structures (in our case the valvular segments) and the hemodynamic variability of the veins is far more complex than that of the arteries. The direct visualization of normal valvular dynamics, transvalvular flow changes (phase transitions, turbulences, and vortices in the sinuses), and reflux has helped to improve and/ or correct classical models. The identification of new types of endovenous injuries (commissural reflux slits, commissural reflux channels, cusp lesions, sliding of the endothelium, endophlebitis plaques, etc) and the understanding of their pathogenesis demonstrate the complexity of living systems. Their simplification and schematization are negative habits arising from a lack of information.
Venous endoscopy may also hold unexpected therapeutic potential. Adapted instruments inserted through the endoscope working channel will allow endoscopic valvuloplasty, minimally invasive embolectomy avoiding valve damage, stent placement, etc, thereby promoting better and faster recovery for patients with chronic venous disease. Using the left subclavian vein path, we have reached the right atrium with an adapted endoscope (in collaboration with Constantin Bătăiosu MD, interventional cardiologist), making possible the correct positioning of pacemaker electrodes in the venous sinus. Interventional cardiology can be carried out using the new generation of faster video cameras, which will eliminate the distortions induced by cardiac motion.
Venous endoscopy can identify normal endoluminal vein structures clearly. The venous valves are specific morphologic structures:
• Truncal valves are located on the deep venous trunks, saphenous trunks, or collateral veins. At the confluence of venous trunks (posterior tibial trunk and tibioperoneal trunk), the junction spur folds gradually creating a well-defined cusp, which, under increased pressure of the perfusion liquid, closes one or other tributary orifice randomly. Under gradually increasing liquid pressure, truncal valve dynamics are not symmetric in the sense that the cusps’ opening process starts at one of the commissural corners, while the closing process is similar but in reverse. It is thus possible to open the valve in order to explore the distal subvalvular segments by projecting an eccentric jet of fluid toward the commissure that opens initially. Instead, applying a high axial pressure makes the valve close tightly.
• Ostial valves are fibrous, strong, and usually bicuspid or—rarely—tricuspid and are located in the saphenous vein estuaries and at the junctions with perforating vein (Figure 1). We have sometimes observed an exuberant valve, with thick, translucent cusps situated at the ostium of the anterior accessory vein of the thigh, and found it very hard to open with a retrograde liquid jet.
• Ridge valves, which we consider to be specific to the deep veins, are located at the confluence of two veins converging to form a sinus. Ridge valves have ample S-shaped cusps, which inflect in opposite directions when the luminal pressure increases, with one half over one drainage orifice and the other half over the other orifice. When the ridge is the drainage spot of a third tributary, the valve consists of two large cusps with a terminal hem with the same S-shape, protecting the central orifice by apposition and the lateral ones by inflecting in opposite directions.1,2 By agile and clever handling of the fluid jet, the cusps can be opened, revealing the ostium of the converging affluent (Figure 2).
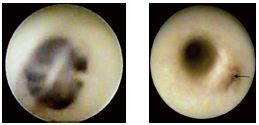
Figure 1. Ostium valve of a great saphenous vein affluent. The
blood is seen through the transparent cusps(A). A great
saphenous vein affluent with a closed ostial valve.(B)
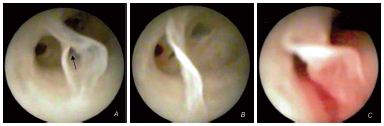
Figure 2. Ridge valve through which a third affluent drains
(A, B). S-shaped ridge valve (C).
• Occasionally the exploration can reveal other endoluminal structures, some of which may be congenital:
– Endothelial cords traversing the lumen, attached to the two opposite walls
– Inconstant endothelial strips, disposed as transversal bars parallel to the skin surface
– Inconstant endothelial folds, which appear to be anchored to the venous wall especially in veins affected by endophlebitis in the past.
Our phleboendoscopic observations have revealed that the deep venous system (DVS) is more valvular than the superficial venous system (SVS) and has a higher number of tributaries. Hammersen’s research has proved that the free edge of the cusps, floating in the bloodstream, is like a curved fold presenting a full-length hem and an icicle-shaped extension (“Zapf”). In our observations, when the hem is obvious, the filamentary extensions are missing. The hem is much more constant than the extensions. Altered, shorter valves have more obvious borders. We believe that the icicle-shaped filamentary extension is also a secondary structure appearing following inflammation of the cusps.
The endothelium is smooth, supple, and of a whitish color. In the great saphenous vein (GSV), the venous wall is perforated by two types of orifices: some are tributaries of the collateral veins, characterized by well-defined valvular cusps, protecting the ostium (Figure 1); others are the emerging ostium of perforating veins where the valves are absent. The ostium has a circular shape, which sometimes looks like a funnel. In the case of reflux, the perforating vein must accept an extra volume of blood. The port of origin will become wider and deeper, with an oval shape caused by the dynamic blood vortex created at the entry of the perforating vein by the suction force (depression force) occurring in the DVS at the time of “muscle diastole” (reentry in the perforating vein). Often, a more obvious semicircular gutter appears at the orifice of the perforating vein (Figure 3).
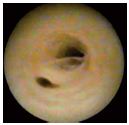
Figure 3. Great saphenous vein with an insufficient truncal
valve and reentry perforator vein with an oval ostium and
reflux-modeled channel.
The valves present under the level of the perforating vein ostium act like an antireflux dam, the reflux volume accepted by the perforating vein being higher. This protects the valve, relieving the increased pressure exerted on the cusps.
The major calf tributaries (anterior and posterior calf saphenous veins) are not always equipped with shedding ostial valves, which may explain their varicose transformation in the case of saphenous reflux.
Low-pressure compression maneuvers on the skin surface can help differentiate between superficial and deep walls and venous margins upon endoscopic examination. When compressing the calf or thigh, insufficient perforator veins can be identified by the appearance of a saphenous blood jet reflux (the blowout phenomenon), which is pushed further distally by the perfusion liquid (Figure 4).
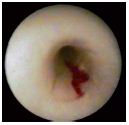
Figure 4. The blow-out phenomenon through an insufficient
perforating vein of the calf.
Videoendoscopy alone cannot identify the venous tributaries. Venous affluents constantly drain the blood on the sides of the collectors. Van Cleef has identified affluents that open onto the collectors into venous sinuses.2,3
The venous tributaries are remarkably valvular. The origin of the popliteal vein often looks like “glove fingers” with a large sinus onto which multiple tributaries open, which confirms previous phlebographic observations.
There are always valves at the confluence of veins, with very well defined, strong cusps with a marginal hem.
Pathological processes, usually inflammatory, alter the valvular system but also the venous wall in its entirety. In certain circumstances, due to persistent hemodynamic disturbances, venous inflammation is prolonged, lasting up to a few years. An example of this is the postthrombotic syndrome, which in our opinion is progressive, with periods of attenuation interrupted by phases of acute venous lesions.
In such a context, the valves, the endothelium, and the whole wall present lesions of varying severity. Their topography is segmental, with maximum severity at the junctions, where the valves are inserted. These valves can remain present as heavily modified and completely nonfunctional artifacts.
Based on our endoscopic observations we have proposed a classification of venous valve lesions.2,4
Classification of valvular lesions
1. Functional valve lesions (type I) due to progressive and prolonged increases in venous pressure. Here we distinguish:
• Commissural reflux slit (type I) (Figure 5),
• Commissural reflux channel (type Ib) (Figure 6).
The difference between these two subtypes is morphological, and is obviously correlated with reflux volume. The eccentric character of these lesions is noteworthy.
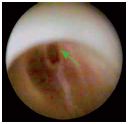
Figure 5. Racket-shaped type-Ia valvular lesion (commissural
reflux slit)
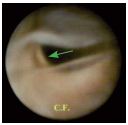
Figure 6. Type-Ib Valvular lesion (commissural reflux channel).
We can observe the threshold of the insertion ring.
2. Traumatic organic valve lesions (type II) – valvular ruptures:
• Commissural: clefts and tears (type IIa) (Figure 7),
• Cusp insertion lesions: linear perforations (type IIb) (Figure 8).
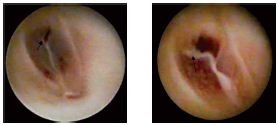
Figure 7. Type-IIa valvular lesions; the valvular rupture is at
the commissure, which reflects the high-pressure stress exerted on
this area of the cusp.
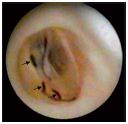
Figure 8. Type-IIb valvular lesions–linear perforations at points
of cusp insertion. The transvalvular pressure loss through those
perforations does not allow for an optimal distension of the
lumen.
These lesion subtypes do not seem to be purely traumatic, with leukocytic infiltration of the cranial surface of the cusps2,5 causing gaps in their structure as well as endothelial cell apoptosis. However, trauma is the most important factor explaining ruptures near the point of insertion of the cusps when the dynamic force of the reflux is very strong. In these cases, the direction of rupture is perpendicular to the direction of the reflux. We have frequently observed valvular tears located at the commissures of the cusps or more rarely at the base of the cusps, causing valvular insufficiency and reflux. The direction of the tear at the commissures is parallel to the axis of the venous segment (dynamic force in the direction of flow). The presence of tears at this level is a strong argument for the presence of a particular hemodynamic stress on the cusps. In keeping with other endoscopic observations, we did not find any significant inflammatory changes on the valves, maybe due to poor blood supply.
3. Inflammatory organic lesions (type III) (Figure 9).
Structural inflammatory rearrangement of the cusps was found only in the context of a broader involvement of the vein wall with altered parietal size, shape, elasticity, etc. Some authors believe that inflammatory valvular alterations are primary5 and solitary, basing their argument on the significantly increased expression of adhesion molecules and the presence of monocytes in the endothelium of the cardiac surface of the cusps.
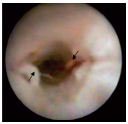
Figure 9. Type-III valvular lesions (chronic inflammatory
alterations of the cusps). Parietal and valvular rearrangement –
valvular vestiges, missing valvular sinus, thrombus situated
under the cusps and adhering to the rigid wall, which has a
narrowed, irregular lumen.
4. Valvular vestiges (type IV) (Figure 10). Here we include valvular debris left by an inflammatory process or iterative reflux trauma. This type of lesion is often and easily confused with endothelial folds or fringes. The venous sinus is absent and the vein is fully tubular.
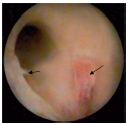
Figure 10. Type-IV valvular lesions (valvular vestiges)
In 1993, Hoshino classified the valvular lesions into three stages: stretched commissures, valve perforations, and valve splitting.6 In 1997, based on endoscopic observations made in 1991, van Cleef proposed a 5-grade classification (from 0-normal valve to 4-severe lesions).2,3
In the absence of valves or valvular vestiges, endoscopy of the GSV cannot really assess the elasticity of the wall, the perfusion liquid being easily drained into the DVS through the perforating veins. This difficulty may be substituted by compression on the venous path distal to the tip of the endoscope, so that we can achieve a strong enough pressure with the perfusion liquid in order to distend the venous wall and ensure good observation. In insufficient, tubular GSVs we have identified a “concentric beach gravel” appearance (Figure 11), which is evidence of the so-called process of “intimal sliding,” a phenomenon that can be anticipated by theoretical hemodynamic studies. Our histopathological observations found that the sliding phenomenon occurs in the internal elastic lamina and is accompanied by ruptures and the development of subintimal vacuoles (Figure 12).
The endothelium may present acute inflammatory lesions of endophlebitis with suggestive erysipelas-like polycyclic margins (Figure 13).
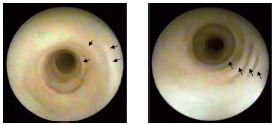
Figure 11. Intimal sliding secondary to the hemodynamic force
of the reflux (“concentric beach gravel” appearance)
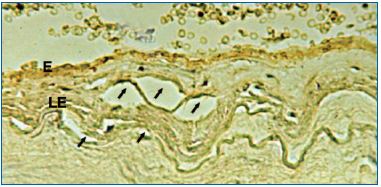
Figure 12. Internal elastic lamina tears with partial intimal
disjunction and underlying vacuolar appearance visualized by
immunohistochemistry.
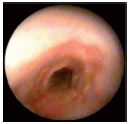
Figure 13. Posterior tibial vein under the confluence with the
popliteal vein showing obvious erysipelas-like endothelitis
Endophlebitis usually appears in the proximity of older lesions. Endoscopy may reveal polyps of various sizes, which can develop either on large venous segments or at the confluence of the tributaries, with a “champagne cork” appearance and which are obviously obstructive and block the passage of the endoscope (Figure 14). It is possible that the starting point of this structure is the development of a thrombus in the ostial valve of the affluent. The discovery of these lesions is strictly related to the introduction of endoscopic examination, as they do not have any clinical symptoms at all.
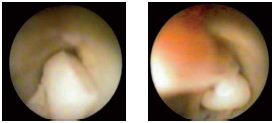
Figure 14. Large endothelial polyps in the venous lumen
Endothelial strips, endothelial folds, and sometimes endothelial flaps can be seen in addition to polyps, all of them representing signs of endophlebitis, after restrictive or obstructive (thrombotic) phlebitic processes.
Sometimes, the venous wall is segmented, modified, rigid, and without flexibility and there are multiple polypoid lesions in the endothelium. In some cases, we found bulky sessile polyps obstructing the lumen.
The most frequent site of thrombosis is the DVS. After a recent thrombosis, the clot ascends several valvular floors, without adhering to the cusps, a minimal increase in the pressure of the perfusion liquid opening the valve so that the thrombus floats freely into the lumen. We often found that recent thrombi, which are rotten cherry–colored, highly elastic and with a diameter of 4 to 5 mm, end up in the lumen of the main sections of collateral affluents. I have personally never observed any recent thrombi originating in the valvular sinuses. However, the presence of a thrombus between the cusps of a valve acts as a wedge, blocking valve closure at successive levels, and opening up a long “path” for reflux. This explains the occurrence of edema and its rapid regression in the supine (reclining) position (Figure 15).
Old thrombi are pale, yellow, or white, sometimes threadlike, and adhere to the vestiges of the valves or to areas of extreme rearrangement of the endothelium (Figure 9).
In postthrombotic syndrome the appearance of the lumen is completely altered, narrowed, almost laminated. We found recanalization always to be situated eccentrically near the wall. The intima is thickened and the endothelial surface is atrophic, irregular, and slightly rough. The lumen is often reduced to a semilunar slit crossed by multiple straps that anchor the thrombus surface to the wall. Sometimes, the venous endothelium is deeply modified, with damaged valves and a reduced and irregular lumen resembling proliferative endophlebitis (Figures 9 and 16).
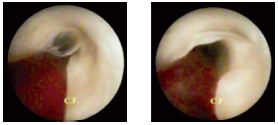
Figure 15. Transvalvular thrombus. Increased perfusion liquid
pressure opens up the valves, followed by multiple level reflux.
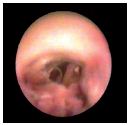
Figure 16. Proliferative endophlebitis. Complete rearrangement
of the endothelium, which has a rough surface.
Lumen alterations are characterized by irregular contours, secondary to fibrous rearrangement and followed by wall hardening. In obstructive post thrombotic syndrome, the lumen is laminated and eccentric. The formation of new channels takes place most often marginally near the wall and, exceptionally, in the center of the thrombus. The trajectory of the new channels is sometimes a twisted path through endothelized thrombus remains, the appearance of which is emphasized in color Doppler examination. In post thrombotic syndrome, the lesions are not stabilized. The potential for progression is high due to an increased risk of reigniting inflammatory lesions, pure phlebitis, and/or thrombosis. Alterations of the endothelium are the trigger. Sometimes, segmental inflammatory acute lesions can be found (areas of endophlebitis with polycyclic slightly elevated margins resembling erysipelas) years after the initial thrombotic event.
Post thrombotic syndrome is by itself a permanent thrombophilia, so that any combination of other predisposing factors, insignificant under normal conditions, can trigger the recurrence of inflammatory phenomena, increase the extent of the lesions, and worsen hemodynamics. Thus, post thrombotic syndrome should be considered as a complex progressive damaging condition requiring lifelong treatment.
In the context of severe chronic ischemic syndromes we have observed extensive total destruction of the venous valves with the venous lumen subsequently becoming tubular and the venous sinuses being destroyed, so that the veins look like arteries.2,7
In conclusion, venous endoscopy is the examination that offers the richest information on the normal and pathological morphophysiology of the venous system of the lower limbs. In addition to its diagnostic value, the fundamental knowledge it has provided has proven useful in the surgical repair of insufficient valves. In the future, early treatment of deep vein thrombosis will benefit from endoscopy performed with new adapted instruments.


REFERENCES
1. Calotă F, Camen D: Atlas of vascular endoscopy of the lower limbs. Craiova, Romania: Sitech ed; 2006.
2. Calotă F, Mogoantă SS. Venous endoscopy in Phlebopatology [in Romanian]. Bucharest, Romania: Romanian Academy Publishing House; 2011:87-95.
3. van Cleef JF. VCT classification (valve, cusp, tributary) and venous endoscopy [in French]. J Mal Vasc. 1997;22:101- 104.
4. Calotă F, Mogoantă SS, Vasilescu MM, et al. The valvular segment of the lower limbs venous system: anatomical, physiological and physiopathological aspects. Rom J Morphol Embryol. 2010;51:157- 161.
5. Ono T, Bergan JJ, Schmid-Schönbein GW. Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998,27:158-166.
6. Hoshino S, Satakawa H, Iwaya F, et al. External valvuloplasty under preoperative angioscopic control. Phlébologie. 1993;46:521-529.
7. Calotă F, Vasile I, Paşalega M, et al. Secondary deep venous lesions in the severe chronic ischemic syndrome of the lower extremities. Rom J Angiol Vasc Surg. 2007;3-4:83-86.
