Treatment options for pelvic congestion syndrome
Santiago ZUBICOA EZPELETA1;
Neil M. KHILNAN2
and Vascular Surgery Unit, Madrid, Spain
2 Division of Interventional Radiology,
New York Presbyterian Hospital, Weill
Cornell Medical College, New York, NY,
USA
Abstract
Pelvic congestion syndrome (PCS) and its main symptom, chronic pelvic pain, are caused by an increase in pressure, number, and caliber of intrapelvic venous structures. These structures are veins with a varicose morphology (ie, tortuous and ectatic with a very retarded flow) that are typically caused by inverted flow in the valveless and enlarged gonadal axis and, in some cases, the branches of the internal iliac tributaries. Once a diagnosis is made in symptomatic patients, embolization of the insufficient varicose veins is the preferred treatment. This endovascular procedure is less expensive than surgery, but it is also less invasive, thereby offering a safe, effective, and minimally invasive treatment option that restores patients to normal. The procedure is very successful in blocking the abnormal blood flow in the majority of cases. Embolization is typically performed using mechanical devices, such as coils, in combination with liquid sclerosants. There are different approaches that will be discussed in this article, according to the need of every case. PCS could also derive from a venous compression, such as that observed in the Nutcracker or May-Thurner syndromes. In these cases, besides using embolization, it might be necessary to place a stent to reopen the affected veins to eliminate the pressure that causes venous hypertension and, consequently, PCS symptoms. Other treatments, such as surgical procedures, sclerotherapy, or the use of drugs, can be utilized as an alternative or a complement to endovascular treatments in selected cases, and these alternatives may contribute to symptom relief.
Introduction
Pelvic congestion syndrome (PCS) is defined primarily as nonmenstrual chronic pelvic pain lasting longer than 6 months that is caused by pelvic venous hypertension. Up to 30% of all patients with chronic pelvic pain have no defined cause for their symptoms. Increasingly, PCS is thought to be responsible for the symptoms in many of these patients. Most of the literature related to the diagnosis and treatment of pelvic venous disorders focuses on pelvic pain. As mentioned in prior sections, pelvic venous disorders can also lead to lower extremity and vulvar varicose veins; some authors have reported on the value of treating pelvic venous hypertension to improve these veins and the patient’s symptoms.1 Although these issues should be considered separately when assessing the outcomes of therapy, they are closely related.
Endovascular treatment of pelvic venous disorders
There are multiple options for the treatment of the pelvic venous disorders. For the most part, the treatments are focused on eliminating the venous reflux that is thought to be the most frequent cause of symptoms. Endovascular therapy is the most commonly used approach. It utilizes venography to confirm the diagnosis of a pelvic venous disorder and to define the refluxing pathways, and it provides the possibility of proceeding with the treatment at the same time as the diagnosis. The combined minimally invasive approach is usually performed as an outpatient procedure with a very low rate of morbidity or complications. Endovascular techniques are very efficient not only to treat PCS derived from reflux, but also to treat PCS derived from compressive etiologies, such as the Nutcracker syndrome (left renal compression between the aorta and the superior mesenteric artery), the May-Thurner syndrome (nonthrombotic compression of the common iliac vein by an iliac artery), or other compressions with different etiologies. Compared with surgery, endovascular procedures are less aggressive for the patient.
The pelvic venous syndromes where increased pressure is caused by venous insufficiency can be treated by occlusion of the insufficient axes by embolization. In contrast, venous hypertension derived from compression can be treated using venous stenting, in addition to treating the insufficiencies.
Embolization
As previously stated, embolization has a low rate of morbidity and complications. The first reported case of embolization of uterine-ovarian varices was described by Edwards et al in 1993.2 Since then, this procedure has frequently been used with positive results in most patients.3-6 The aim of embolization is to occlude insufficient venous axes as close as possible to the origin of the leak. In pelvic venous disorders, these axes will be the gonadal axes, pelvic varicose veins, and insufficient tributary branches of the internal iliac veins.
The procedure starts with patients in a supine position on the radiological tilt table. One possible approach is to use the right basilic vein at the elbow for venous access. Patient acceptance of this access point is probably higher, the risk of significant arterial or lung injury is low, and immediate ambulation following recovery from any sedation is possible. Alternatively, the internal jugular vein could be used, although patients are more skeptical about the safety and comfort of this access point. However, with ultrasound guidance, the internal jugular vein access point is very safe, and the procedure is well tolerated. In addition, catheter choices, venous catheterization, and catheter exchanges are simpler because the pathway to the pelvic vein is shorter and straighter. When it is necessary to access a retroaortic left renal vein or in cases where catheter access to the gonadal axis is difficult to obtain from the superior cava vein, the femoral vein can be used as an access point.
A renal and left iliac venogram is usually the first step in the evaluation of compressive syndromes, which is done using fluoroscopic-controlled guidance and a multipurpose catheter (80-125 cm, 4-5 F) to measure the pressures between the renal and left iliac veins and the inferior vena cava. Significant left renal and left common iliac vein lesions are identified by either significant compression of the vein, opacification of the collateral veins, or retrograde flow through the gonadal veins. The next step is to cannulate the gonadal and internal iliac veins selectively. The left gonadal vein is a tributary of the left renal vein, and catheterization of this vein is usually done first. The right gonadal vein is a tributary of the inferior vena cava just below the right renal vein, and it is often catheterized next. Contrast medium is injected into the vessel of interest, and the patient (instructed before the procedure) is asked to perform a Valsalva maneuver so that the doctor can check for reflux. Tilting the table into a reverse Trendelenburg position can aid in this evaluation. Retrograde flow toward the pelvis is diagnostic of reflux. Incompetent gonadal veins are generally dilated (>6-8 mm in diameter) and the contrast generally pools in the pelvis after the injection.7 When an abnormal vein is encountered, treatment is usually performed before evaluating the next vein (Figure 1). The internal iliac veins are subsequently studied using venography. In the internal iliac veins, the Valsalva maneuver will be very helpful to identify insufficiency and to define escape points to the lower limbs. In cases where such reflux is found, it is very important to occlude these vessels to avoid incomplete clinical improvement.
Those axes showing significant caliber increases and retrograde venous flow that fills varicose-like venous veins should be occluded using the same catheter that has already been placed. The embolization material used has varied in the literature, and no evidence-based recommendation can be made about the best approach. Most of the literature has reported using gonadal vein occlusion with mechanical devices, whereas other reports inject a sclerosant into the varicose veins. At this point, no report on the efficacy of occluding the abnormal pelvic venous plexus alone without occluding the gonadal veins has been reported.
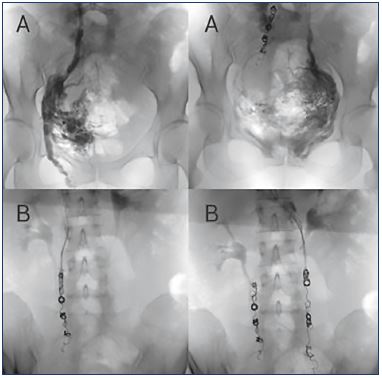
Figure 1. Reflux of gonadal veins can be treated by embolization.
Panels A. Pelvic selective phlebography showing insufficient
gonadal veins in the same patient (left and right gonadal
veins). When a gonadal vein is refluxive, contrast media flows in
a retrograde direction when injected into the proximal portion
of the vein and even fills varicose uterine veins.
Panels B. Assessment in the same patient after embolization
using controlled released (left and right gonadal veins). It is
important to occlude all insufficient axes by covering the entire
vessel to avoid recidivism.
Embolization is typically performed using a “sandwich”- mixed technique, which combines metallic devices with 2% ethoxisclerol foam (prepared according to Tessari’s method during the procedure).8 The treatment is extended proximally to 5 cm from the origin of the gonadal veins off the left renal vein or inferior vena cava, taking care to close all the potential collateral points, either by direct catheterization and embolization or by covering with coils.
An alternative, the embolization could also begin by injecting a foam or liquid sclerosant (generally sodium tetradecyl sulfate mixed with contrast to result in a 1% to 1.5% concentration) as distally as possible to occlude the pelvic venous plexus using an occlusion balloon just above the true pelvis where the tributaries of the main ovarian vein join. Before sclerosis, the volume of the varicose pelvic venous plexus can be estimated by injecting contrast with the balloon inflated until normal veins start to be opacified. Generally, sclerosis is performed with a volume of sclerosant that is about 75% of the measured volume. After a few coils are placed in the lower part of the ovarian vein, the balloon is deflated and withdrawn a few centimeters, and the sclerosant injection is repeated utilizing a similar sandwich technique until about 5 cm from the termination of the gonadal vein.
To close the gonadal veins, mechanical devices, such as coils of different sizes and plugs, have been used, in combination with different sclerosants. The mechanical devices are safe and efficient, and they are the best option to obtain a complete and durable occlusion. It is desirable to use coils that are 40 to 50 mm in length to cover the entire course of the axis, avoiding the possibility of derivations by collateral systems or anatomic variations. In some cases, segmental mechanical embolization is desired to limit the expense of the coils used. These coils must be of radiopaque materials to allow for fluoroscopic control. The use of controlled-release detachable coils can minimize the potential for migration due to under sizing and allows relocation to occur.
Following gonadal-vein closure, internal iliac veins should be investigated and, if necessary, treated. In the presence of leak points to the lower limbs or connections of internal iliac veins with lower periuterine varicose veins, selective cannulation and embolization with coils and/ or sclerosants should be performed.9 It is important to eliminate all abdominal-pelvic refluxes to minimize the risk of inadequate treatment or recurrence of PCS symptoms. The use of controlled-release detachable coils can be very helpful in those axes to assure that they are placed in the desired location and to avoid migration to a pulmonary artery. Also, it is possible to use an occlusion balloon to find abnormal appearing veins on diagnostic venography and to use the occlusion balloon to sclerose the abnormal components of the pelvic venous plexus as previously described. In men, embolization is used to occlude the left and, less often the right, spermatic vein, vas deferens, and cremasteric vein, thus solving the male varicocele that causes similar symptoms to PCS.
Patients often experience a “postembolization syndrome” after pelvic vein embolization, which is characterized by mild-to-moderate pelvic pain and fever.1 These symptoms last a few days and can be treated with analgesics. Besides this syndrome, other less common complications have been documented in the literature, such as coil migration to the pulmonary artery or into the left renal vein. However, the successful recovery of a misplaced coil has been described using an Amplatz loop snare.
Clinical outcomes are monitored after embolization with clinical follow-up visits and a transvaginal Duplex ultrasound at 1, 3, 6, and 12 months postprocedure.
Pelvic leaking point to lower limbs
Pelvic-derived lower extremity varicose veins are found in up to 20% of women with varicose veins.10,11 The prevalence might be even higher in populations with persistent or recurrent varicose veins after previous treatment.12 Pelvicderived lower extremity varicose veins result from pelvic venous hypertension that escapes to the legs through one of four common points. The most common escape point is the perineal or P point, where the internal and external pudendal veins connect in the urogenital triangle. These leaking points can lead to inner thigh and posterior labial varicose veins. The next most common escape point is the inguinal or I point. At this location, pelvic venous plexus– derived reflux passes through the external inguinal ring via a recanalized vein of the round ligament, emerging in the groin medial to the common femoral vein. This can lead to groin and labial varicose veins (Figure 2). Other less commonly discussed escape points include the gluteal points and varicose veins traveling along the sciatic nerve (Figure 3).
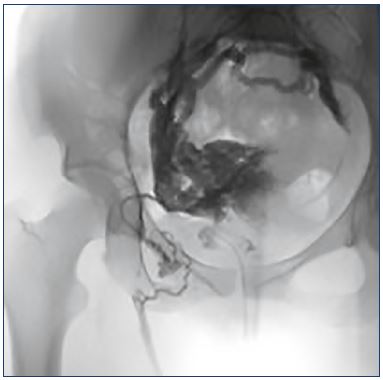
Figure 2. Pelvic leaking point to lower limbs through a round
ligament vein.
Pelvic congestion syndrome could affect the lower limbs, which
is true in this example of an insufficient right gonadal axis that
connects with the uterine-ovarian plexus, making those veins
insufficient, and through the right round ligament vein (with a
characteristic “C” shape) that connects with the great saphenous
vein as a paraostial leak.
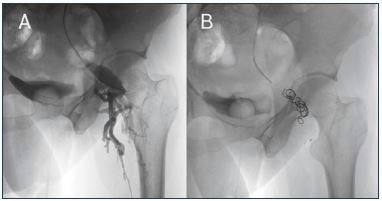
Figure 3. Pelvic leaking point to lower limbs through the gluteal
vein.
An insufficiency in the internal iliac axis could leak to lower limbs
through the gluteal-ischial axis as is observed in this patient.
Panel A. The catheter should be placed as distal as possible
in the left internal iliac vein, and when using the Valsalva
maneuver, the contrast flows through the left gluteal vein to the
left limb through a repermeabilized postaxial axis.
Panel B. After embolization with coils, no contrast is observed in
the gluteal axis because the insufficiency has been corrected.
There are few data on treating pelvic-derived lower extremity varicose veins with pelvic embolization. It is important for physicians to recognize pelvic-derived varicose veins and to consider the value of addressing the pelvic venous hypertension in each case. In patients with both chronic pelvic pain and clinically significant pelvic-derived varicose veins, pelvic embolization is usually done first. Patients with such veins usually require treatment of their leg veins to treat lower extremity symptoms or they require treatment for aesthetic reasons after an embolization is done. In patients with pelvic-derived varicose veins, but no significant vulvar or pelvic symptoms, an alternative approach is to begin by treating the leg veins with contemporary venous treatments in addition to sclerotherapy to treat the pelvic-derived varicose veins from just below the escape points, and then see how the patient responds. Patients with a good clinical response to such treatment may avoid pelvic embolization. If the varicose veins recur early or if the lower extremity symptoms do not resolve after such treatment, embolization can be done afterward.
Sclerotherapy of these veins can be done with visual, transillumination, or ultrasound guidance. With the visual sclerotherapy approach, the amount of sclerosant injected into each vein is empiric and it is generally between 0.5 and 2 mL of an appropriate concentration of 1% polidocanol or 0.5% to 3% sodium tetradecyl sulfate using either liquid or foam. Fluoroscopic-guided sclerotherapy using a mixture of contrast and sclerosant as previously discussed can be done with the advantage of being able to titrate the drug dose to obliterate the connections up to the pelvic venous plexus. Such venographic guidance, analogous to that used to treat venous malformations, allows the operator to identify opacification of normal veins, thereby enhancing safety. The injection can be stopped at that point or continued by manually compressing the connections to a normal vein or manually compressing to direct the sclerosant to the desired targets. Although little evidence has been published to support these approaches; anecdotally, these approaches are effective and durable for patients without significant chronic pelvic pain.
Stenting
Venous compression syndromes could also lead to pelvic venous plexus hypertension and result in PCS. In these cases, placing a stent can treat the compression. A stent is a permanent intravascular device that is placed into the vein using a catheter to reopen narrowed or occluded blood vessels. The stents used in the venous system are usually generally self-expandable and variable regarding the material used and the design of the braids. The stents come in multiple diameters and lengths, and they are selected based on the morphology of the treated vessels and extent of the lesions.
Nonthrombotic compression of the left common iliac vein by the right common iliac artery, often referred to as the May- Thurner syndrome, can also lead to pelvic venous plexus hypertension. Placement of a stent in the left common iliac vein can be performed by a left common femoral vein puncture. The desirable stent would have great radial strength and a high precision placement. Placement of a venographic catheter in the inferior vena cava via a simultaneous right common femoral vein access allows venography to visualize the inferior vena cava confluence, helping to ensure a precise stent placement.
In general, for left iliac vein stenting, the use of self-expandable devices is recommended, in part because balloon-expandable stents are more painful for patients. The most commonly used stent in this location is the self-expanding Wallstent because they are larger than other self-expanding stents. The size used will vary between 16 and 20 mm. A balloon-expandable stent, such as the Palmaz-Genesis series with high radial strength, is utilized in some cases. The recent development of dedicated venous stents with certain desirable properties, such as the Veniti, Sinus Venous, and Zilver stents may change the choice of stents for this lesion as more data on their performance is collected (Figure 4, Panels A and B). The placement of a stent in the iliac vein can eliminate any pressure gradient to venous flow, and it can correct the main PCS symptoms by decreasing the pressure of the collateral flow directed through the pelvic venous plexus. Preliminary data suggest that this treatment of the stenosis may be more important to eliminate pelvic pain than treating concurrent reflux in the gonadal vein if they coexist.13
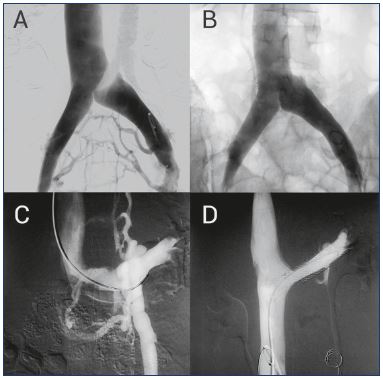
Figure 4. Treating compressive syndromes with stenting.
Panel A. May-Thurner syndrome or compression of left iliac vein
is shown in this pelvic phlebography. The presence of collateral
pathways in the uterine and pelvic plexus is responsible for the
PCS symptoms.
Panel B. After placing a stent, normal blood flow is reestablished,
avoiding pathologic collateral systems.
Panel C. Nutcracker syndrome or left renal vein compression
is shown in the pelvic phlebography. In this case, the pressure
increase develops an insufficiency in the left gonadal vein,
with the concurrence of an ascending paravertebral collateral
system.
Panel D. The left renal vein compression is corrected with a
stent, which again reestablishes normal blood flow.
The Nutcracker or mesoaortic syndrome is defined as compression of the left renal vein between the superior mesenteric artery and the aorta (or the aorta and the lumbar spine in the case of a retroaortic left renal vein). Patients with this condition can present with low back or left flank pain and micro- or macrohematuria. Increasingly, it has been recognized that the Nutcracker syndrome can lead to pelvic venous hypertension and chronic pelvic pain by diverting the venous drainage of the left kidney into the pelvis.
Clinical evaluation of patients with chronic pelvic pain and suspected PCS often includes an ultrasound. Duplex ultrasound evaluation of the left renal vein can identify this stenosis by measuring and comparing the diameter and the velocity of flow in the narrow portion and the hilum portion of the vein. Since it is less invasive than the currently available surgical options, it is natural to consider placing a stent in the left renal vein to treat this entity.4 Since the stent delivery catheter is rigid, thicker, and often shorter than a multipurpose catheter, stenting is performed through the right jugular vein or femoral vein, according to the angle and morphology of the left renal vein. Since the length of the renal vein is short, the risk of stent migration to the pulmonary artery is much higher than in many other locations where venous stents are placed. In addition, the angle of the renal vein and inferior vena cava adds to the challenge of accurately placing a stent. Due to the risk of migration, placing a stent in the left renal vein is not universally accepted using currently available stents. Some experienced physicians who are very skilled with endovascular techniques still think a minimally invasive open surgical approach (in which the left renal vein is transposed lower down on the inferior vena cava with or without a venous cuff, which frees up space between the aorta and superior mesenteric artery) is superior to stenting.
The typical stent choice is a stainless steel self-expandable Wallstent. Proper placement of the stent requires the use of a stent with sufficient caliber as well as avoiding excessive protrusion in the inferior cava vein or into the renal hilum branch veins. If during the release, misplacing is observed, it is possible to resheath the Wallstent device to relocate it properly. The most used stent for the left renal vein is 14 mm in diameter and 40 to 60 mm in length (Figure 4, Panels C and D).
A postprocedure venogram should demonstrate normal flow from the left kidney to the inferior vena cava, without reflux into the gonadal vein or opacification of collateral perirenal veins. Finally, it is desirable to measure pressures before and after the procedure to ensure the normalization of any measured gradient to confirm success, although these pressures are quite low even in patients with severe compression.
Other treatments
Besides the endovascular techniques previously described, there are alternative methods for treating PCS, such as drugs and surgery. These therapies are less frequently used because of their disadvantages compared with embolization, such as a short-term resolution of symptoms or high invasiveness.
For reflux disease, other treatment options include open or laparoscopic surgery to ligate the insufficient veins. However, these procedures are rarely performed because they are more invasive than endovascular embolization procedures, they require a general anesthetic, and the recovery period is longer. Given that surgical ligation can only interrupt the refluxing pathway at a limited number of locations, recurrences may be more common. In many occasions, patients need other complementary procedures, such as sclerotherapy, to correct external leaking points or lower limb varicosities that are derived from PCS. In addition, the use of compression stockings may be desirable to prevent the occurrence or further progression of venous disorders.
Alone, medical treatment is not considered to be valuable for treating PCS. Treatment with medroxyprogesterone acetate or the gonadotropin-releasing hormone analog goserelin acetate may provide short-term relief of symptoms; however, recurrence is common and therefore not typically used for long-term care. Phlebotonics are often used, and they act as modulators of the inflammatory response that is responsible for the pain. However, their value in PCS has not been evaluated.
REFERENCES
1. Monedero JL, Ezpeleta SZ, Grimberg M, Correa LV, Gutierrez AJ. Subdiaphragmatic venous insufficiency: embolization treatment using mixed technique. Phlebolymphology. 2004;45:269-275.
2. Edwards RD, Robertson IR, MacLean AB, Hemingway AP. Case report: pelvic pain syndrome–successful treatment of a case by ovarian vein embolization. Clin Radiol. 1993;47:429-431.
3. Capasso P. Ovarian vein embolization for the treatment of pelvic congestion syndrome. Part I: background, anatomy and etiology. Intervention. 2000;4(3):67- 72.
4. Scultetus AH, Villavicencio JL, Gillespie DL. The nutcracker syndrome: its role in the pelvis venous disorders. J Vasc Surg. 2001;34(5):812-819.
5. Scultetus AH, Villavicencio JL, Gillespie DL, Kao TC, Rich MN. The pelvic venous syndromes: analysis of our experience with 57 patients. J Vasc. Surg. 2002;36(5):881-888.
6. d’Archambeau O, Maes M, De Schepper AM. The pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatment. JBR-BTR. 2004;87(1):1-8.
7. Black CM, Thorpe K, Venrbux A, et al. Research reporting standards for endovascular treatment of pelvic venous insufficiency. J Vasc Interv Radiol. 2010;21(6):796-803.
8. Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27(1):58-60.
9. Kim HS, Malhotra AD, Rowe PC, Lee JM, Venbrux AC. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiology. 2006;17:289- 297.
10. Marsh P, Holdstock J, Harrison C, Smith C, Price BA, Whiteley MS. Pelvic vein reflux in female patients with varicose veins: comparison of incidence between a specialist private vein clinic and the vascular department of a National Health Service District General Hospital. Phlebology. 2009;24:108-113.
11. Malgor RD, Labropoulos N. Pattern and types of non-saphenous vein reflux. Phlebology. 2013;28(suppl 1):51-54.
12. Monedero JL, Ezpeleta SZ, Castro JC, Ortiz MC, Fernandez GS. Embolization treatment of recurrent varices of pelvic origin. Phlebology. 2006;21:3-11.
13. Daugherty SF, Gillespie DL. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2015;3(3):283-289.

