Tissue fluid pressure and flow in the subcutaneous tissue in lymphedema – hints for manual and pneumatic compression therapy
Waldemar L OLSZEWSKI1,2,3
Pradeep JAIN4
Govinda AMBUJAM4
Marzanna ZALESKA1
Marta CAKALA1
Tomasz GRADALSKI5
1. Department of Surgical Research and
Transplantology, Medical Research Center,
Polish Academy of Sciences, Warsaw,
Poland
2. Department of Gastrointestinal and
Transplantation Surgery, Central Clinical
Hospital, Ministry of Internal Affairs,
Warsaw, Poland
3. Rikshospitalet / Norwegian Radium Hospital,
Oslo, Norway
4. Indian Lymphology Centers, BHU Varanasi
and TMC Thanjavur
5. Lymphedema Clinic St Lazarus Hospis,
Krakow, Poland
ABSTRACT
Physiotherapy of lymphedema requires knowledge of: a) how high external pressures should be applied manually or set in compression devices in order generate tissue pressures high enough to move the fluid to the non-swollen regions and b) how to measure the tissue fluid flow.
We measured tissue fluid pressure and flow under the skin in the subcutaneous tissue of lymphedematous limbs stage II to IV at rest and during manual and pneumatic compression under various pressures and sleeve inflation timing.
In obstructive lymphedema of lower limbs tissue fluid pressures in the subcutaneous tissue was 2.5±3.0 mmHg (range-1 to +10 mmHg) and did not differ from those measured in normal subjects. During manual massage the applied force generated pressures ranging from 60 to 120 mmHg. Pneumatic compression generated tissue fluid pressures depending on sleeve inflation pressures, however, they were on the average 20% lower than in the inflated chambers. The high pressure gradient across skin and subcutis could be explained by skin rigidity (fibrosis), low hydraulic conductivity of subcutis and dissipation of the applied force in subcutis to the proximal noncompressed regions. Strain gauge put around the limb provided data on girth changes during compression and allowed to calculate the approximate volume of the proximally displaced fluid. It showed that tissue fluid flow occurred during manual compression only during pressing of tissues to stop immediately after its cessation. In contrast, pneumatic sequential compression produced unidirectional flow toward groin without backflow. The total proximally displaced volume from ankle to groin was up to 100ml/cycle.
The obtained data should be useful for physiotherapy allowing to set the manual or pneumatic compression parameters at levels corresponding to the physiological conditions.
INTRODUCTION
Treatment of lymphedema of limbs is a combined modality comprising administration of medicines,1,2 compression procedures based on manual and pneumatic massage,3 wearing elastic stockings or bandages preventing accumulation of fluid and growth of expanded skin and subcutis, also enhancing the efficiency of muscular pump,4 and in selected cases lymphovenous shunts,5 liposuction6 and debulking of abundant tissues.7
The mechanism of action of administered antilymphedema medicines as diosmins and related drugs as well as indications for surgical procedures have been well defined and the results of treatment can be quantified. In contrast, compression procedures bring about diverse effects because of differences in manual massage protocols, pneumatic devices and poor knowledge what pressures and compression timing should be used. There is no information in the literature on mobile tissue fluid pressure and flow in human skin and subcutaneous tissue under normal conditions and in various types of lymphedema. We previously measured lymph pressures and flow in human calf collectors, however, the obtained data did not give insight into the hydraulic conditions in the interstitium8 In lymphedema most lymphatic collectors do not contract as they have a fibrotic wall and are partially obliterated. The bulk of the capillary filtrate accumulates in tissue spaces.9
Compression therapy partly supports, partly replaces the force necessary for propelling tissue fluid toward the root of the limb. Unfortunately, this treatment modality has so far not been based on the knowledge of hydraulic conditions in the interstitium of skin and subcutaneous tissue and blood perivascular spaces. The technical parameters of pneumatic compression devices are based on blood rheological data derived from textbooks. These are the capillary and venous blood pressure, venous blood flow and capacitance and capillary filtration. Unfortunately, there is little analogy between the blood and lymphatic system rheology at the tissue level. Pressures applied by pneumatic compression are set arbitrarily, because of lack of knowledge of skin and subcutis compliance (fibrosis), exact sites of lymphatic obstruction and accumulation of excess of tissue fluid as well as tissue fluid pressures and flow. The recommended compression pressures for pneumatic massage are around 50mmHg, timing of sleeve chamber inflation is usually 5 seconds and it is followed by a rapid deflation. Total compression time by an 8-chamber sleeve usually ranges between 20 to 40 seconds. It is not known whether this timing is sufficient to overcome tissue hydraulic resistance and move the stagnant tissue fluid.
What needs to be elaborated for clinical practice is how much external pressures should be applied either manually or by compression devices in order generate tissue pressures high enough to move the fluid to the non-swollen regions and how to measure the tissue fluid flow.
In this study, we measured tissue fluid pressure and flow under the skin in the subcutaneous tissue of lymphedematous limbs stage II to IV at rest and during manual and pneumatic compression under various applied pressures. The obtained data should be useful for physiotherapy and allow to set the compression parameters at levels corresponding to the physiological conditions.
MATERIAL AND METHODS
Patients.
Study was carried out on 25 patients with lymphedema of one lower limb, stage II to IV, duration of 2 to 15 years. Edema developed month to years after small foot abrasion, insect bite or soft tissue trauma. In 50% of cases edema was complicated by 1 to 3 attacks of dermato-lymphangio-adenitis. In 3 patients edema developed without any detectable reason. Cases with acute inflammation were excluded from the study. Five male volunteers with healthy legs served as controls. Staging was based on the level of edema starting from foot to groin, degree of skin keratosis and fibrosis and soft tissue tonometry.9 Evaluation of lymphatic pathways was done on lymphoscintigraphic pictures. Color Doppler investigation was also carried out to exclude cases with the postthrombophlebitic changes. Calf and thigh circumference were measured in supine position 15 cm below and 15 cm above the lower edge of patella. The study was approved by the Warsaw Medical University and the Indian Universities ethics committees.
Tissue fluid pressure measurement
The wick-in-needle technique was used. An 8 gauge injection needle with a polyethylene tubing (OD 1.34 mm) containing glass-wool wick protruding from the tubing tip at 5mm was introduced under the skin. The outer part of tubing was led outside the compression sleeve, connected to the pressure transducer (Honeywell, Elblinger, Poland) and recording was done using a 3 channel device, pressure range -20 to 150 mmHg (Telsoft, Warsaw, Poland) and LabView software (National Instruments, Austin, TX, USA). The position of transducer was zeroed and pressure recording was started simultaneously with manual compression or sequential inflation of sleeve chambers. The data were collected using Microsoft Excel program and were presented graphically on a pressure/time scale.
Continuous limb girth (volume) measurement
Strain gauge plethysmography was used to measure girth changes in the calf and thigh. The plethysmograph (Hokanson, Bellevue, WA, type EC6) recording vein mode was applied. The girths of mid-calf and mid-thigh were measured and a 2 cm shorter mercury strain gauges were put around limb at chamber levels 3 to 8. Elongation was read off on the recorder graph scale in mm as a change in limb circumference. Inflation of chambers located distally to the strain gauge propelled mobile tissue fluid in proximal direction. Once fluid reached the limb region with strain gauge, its volume increased and so did the girth. Increase in girth was recalculated into volume by multiplying cross area of limb at the studied level by the length of the compressing chamber. Subtracting the volume value before compression from that during compression provided data on the transferred volume.
Manual massage
Limb massaging started distally from the level of pressure sensor and strain gauge, slowly approached them, then it was continued over the site of recording in proximal direction to the groin. Tissue fluid pressure and girth changes were recorded continuously.
Pneumatic compression appliance
We used such a device designed for us by Biocompression (Moonachie, NJ). It was built of 8 segments 9 cm long each, sequentially inflated, inflation pressures were regulated from 50 to 125 mmHg, gradient pressures was proximally decreasing by 20%, inflation time of each chamber was 50 sec, there was no deflation of distal chambers, the deflation time at the end of the cycle was 50 sec. The long inflation time was based on our observations that manual squeezing of tissue fluid from the wound during debulking surgery took a minimum of 50 to 100 sec. No deflation prevented venous return to the distal parts of the limb and venous stasis with increased filtration rate. Sites of pressure and flow recordings have been shown on Figure 1.
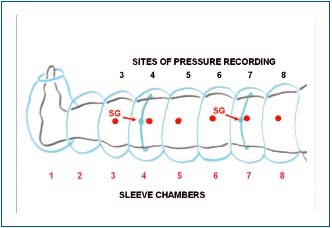
Figure 1. Schematic presentation of lower limb in a pneumatic
sleeve with 8 chambers 9 cm wide each. Tissue fluid pressure was
measured at 6 points indicated by large dots. The lines encircling
calf and thigh show the site of strain gauge location for continuous
measuring of girth changes.
RESULTS
Subcutaneous tissue fluid pressures at rest
Tissue fluid pressure measured under the skin in lymphedematous calfs ranged between – 1.5 and 10 mmHg (mean 2.5±3.0) and in controls between -1.8 and 3.0mmHg (mean 0.8±1.2) (Figure 2). There were no statistically significant differences between the groups. These reading corroborate our previous clinical and experimental findings.10,11 The mechanism of low pressure can be explained by high skin compliance in the early stages of edema formation leading to expansion of the subcutaneous space absorbing excess of capillary filtrate not drained away by lymphatics.
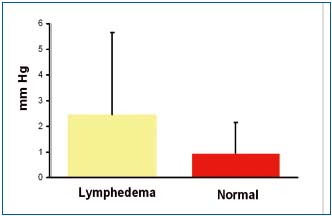
Figure 2. Tissue fluid pressure measured under the calf skin in a
horizontal position in 15 patients with lymphedema stage II to IV
and 5 healthy subjects. No significant differences between
lymphedema and normal limbs. There were no differences
depending on the stage of the disease. Data are means +-SD.
Subcutaneous tissue fluid pressures and flow during manual massage
The manual force for massage is set arbitrarily and never seems to be too high. However, our measurements showed that hands generated tissue fluid pressures ranging as high as 140 mmHg (Figure 3). This was observed in stages II and III. In stage IV high skin rigidity limited the force transfer to subcutis and lower pressures were observed. To investigate whether the manual force is transferred to proximal regions of the limb, we measured pressures at different distances from the massaging hand. Compression 3 cm from the sensor did not show any transfer pressure through this distance . This could be explained by skin rigidity and low hydraulic conductivity of subcutis.
Tissue fluid flow during manual massage was seen only during compression of tissues, to stop immediately after removal of the pressing hand (Figure 4). The calculated flow per compression values were around 5 ml. The skin indentation by the massaging hand usually disappeared within 10-15 sec. This indicated that the displaced fluid returned to its site of origin.
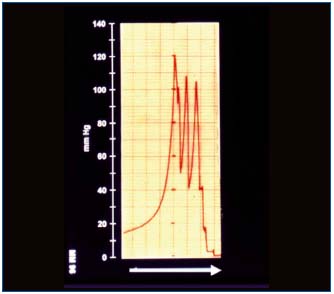
Figure 3. Tissue fluid pressure measured under the skin in a
lymphedematous calf in a horizontal position during manual
massage. Note the short-lasting high pressure waves at each
manual compression and zero level immediately after stopping of
massage. The blinded physiotherapeutist used such a force during
each massage session.
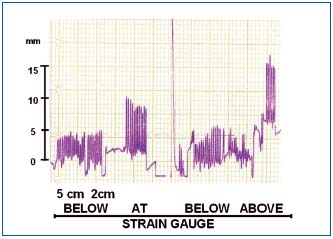
Figure 4. Change in circumference (volume) in a
lymphedematous calf during manual massage recorded at two
levels 10 cm apart. Note that hand compression caused only a
short-lasting increase in circumference with immediate return to
the initial level. There was no increase of circumference when the
massaging hand was put 5 and 2 cm away from the strain gauge.
Pressing tissues directly at the site of strain gauge brought about an
increase in circumference with a fast drop after hand removal.
Pressing above the strain gauge caused increase of circumference
below. This indicated retrograde flow of fluid. 15 mm on scale =
5 mm of calf girth increase = 10-15 ml of calculated tissue fluid
flow.
Subcutaneous tissue pressures and flow during pneumatic massage
The pressures generated in tissue fluid during the first inflation of asleeve chamber were in all cases lower than those in the chamber itself. (Figure 5, 6A, 6B). This high gradient was most likely caused by skin rigidity (fibrosis) and dissipation of the applied force to the proximal noncompressed regions. Interestingly, there was also little tissue fluid pressure transmission in subcutis from the compressed to the non compressed proximal segments for a distance of 9 cm (width of the chamber). In advanced stages IV limited pressure transmission in proximal direction could be seen. An interesting finding was building up pressure in the distal parts of the limb during sequential inflations of proximal chambers. This could be due to flow obstruction at the inguinal level. We also noticed that tissue fluid pressures reached lower levels in the popliteal and upper thigh than in other limb regions. These two regions are usually less swollen and slowly accumulate fluid translocated during sequential massage. Summarized data of 15 patients have been presented on Figure 7.
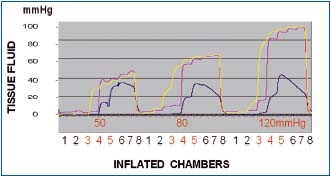
Figure 5. Tissue fluid pressure recording in a normal calf
subcutaneous tissue at the level of compression sleeve chambers 3,
4 and 5. Inflation pressures 50, 80 and 120 mmHg. Inflation time
of each chamber 55 sec, no deflation. Number 1 to 8 denote
consecutive sleeve chambers. Pressures recorded under chambers 3
(yellow line), 4 (red line) and 5 (blue line). Note that pressures
were always lower than those in chambers. Inflation of chambers
1,2 and 3 to 50 mmHg did not generate pressures at level 3.
Inflation of chamber 4 produced pressure of 30 mmHg at level 3
stepwise rising during inflation of consecutive chambers to 45
mmHg. Inflation of chamber 4 also produced tissue fluid pressure
rise to 40 mmHg and of chamber 5 to 12 mmHg, to increase
during inflation of consecutive chambers to 45 and 38, respectively.
Similar pressure curves were observed during inflation of
chambers to 80 and 120 mmHg. Blue line (level 5) represents
pressure at the medial aspect of calf just below the knee usually
with less edema.
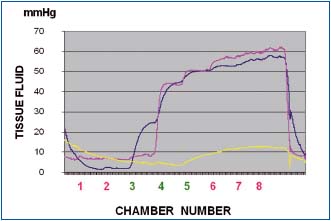
Figure 6a. Tissue fluid pressure in the calf lymphedematous
subcutis stage III during pneumatic compression of 50 mmHg
(inflation time of each chamber 55 sec, no deflation). Pressures
recorded at level 3 (blue line), 4 (red line) and 5 (yellow line). Note
that tissue fluid pressures during first inflation of chambers at
level 3, 4 and 5 were lower than those in the chambers themselves.
Inflation of chambers 1 and 2 to 50 mmHg did not generate
pressures at level 3. Inflation of chamber 3 produced at level 3
pressure of 25mmHg (blue), stepwise rising during inflation of
consecutive chambers to 55 mmHg. Inflation of chamber 4
produced tissue fluid pressure rise at level 3 and 4 to 45 mmHg to
increase during inflation of consecutive chambers to 60. The yellow
line represents pressure the medial aspect of calf just below the
knee (level 5) usually with less edema and low flow resistance.
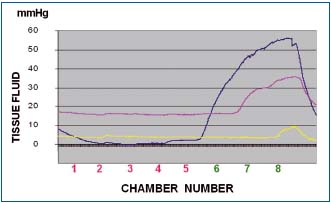
Figure 6b. Tissue fluid pressure in the thigh lymphedematous
subcutis stage IV during pneumatic compression of 50 mmHg).
Pressures recorded at level 6 (blue line), 7 (red line) and 8 (yellow
line). Note that as in the calf, tissue fluid pressures during first
inflation of all chambers were lower than those in the chambers.
Inflation of chambers 1 to 5 to 50 mmHg did not generate
pressures at level 6. Inflation of chamber 6 produced at level 6
pressure of 35 mmHg (blue), stepwise rising during inflation of
consecutive chambers to 55 mmHg. Inflation of chamber 7
produced tissue fluid pressure rise at level 6 to 50 mmHg and at
level 7 to 30 mmHg to increase during inflation of consecutive
chambers to 55 and 35 mmHg. The yellow line represents pressure
at level 8 close to the groin usually with less edema and low flow
resistance.
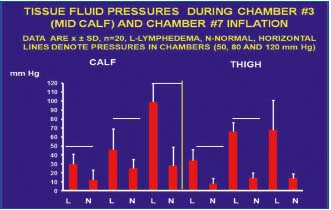
Figure 7. Summarized data of tissue fluid (TF) pressures in calf
and thigh subcutaneous tissue in patients with lymphedema
(n=15) and healthy subjects (n=5) during the first inflation of
pneumatic sleeve to 50, 80 and 120 mmHg. L- lymphedema,
N- normal, horizontal line denotes pressure in the sleeve, mean
values ± SD. Note that TF pressure was in all cases lower than in
the sleeve both in the lymphedema cases and healthy subjects (for
explanation see text). There were also evident differences between
lymphedema and control cases.
Circumference (volume) changes during pneumatic massage
Continuous recording of circumference changes during sequential compression gave indirect insight into the volumes of fluid translocated from the compressed segments to the proximal ones. Following inflation of a chamber, increase of limb circumference occurred proximally to this chamber. Sequential inflations of chambers from 1 to 8 resulted in stepwise increase of circumference in consecutive segments of the limb (Figure 6). The increase in circumference at each level was recalculated into increase in volume. Summarized data of 15 patients are presented on Figure 7. The calculated proximally transferred volume was evident in the calf but much more in the thigh containing large volumes of fluid and fat.
Continuous recording of circumference changes during sequential compression gave insight into the volumes of fluid translocated from one pressed segment to another. Following inflation of a chamber there was increase of circumference in limb segment above this chamber. When the segment with strain gauge was compressed, the girth decreased. Further sequential inflation of proximal chambers brought about girth decrease (Figure 8). This was observed in cases with low resistance to tissue fluid flow. In case of flow obstruction, girth rose to high values. The increase in girth was recalculated into increase in volume. The total volume accumulating fluid volume in the proximal parts of the limb could also be calculated. Summarized data have been presented of Figure 9.
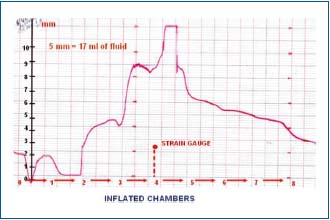
Figure 8. Circumference changes in lymphedematous calf stage
IV during sequential compression at 120 mmHg. Strain gauge
underneath chamber 4. Inflation of chamber 1 brought about
decrease of girth due to halting of venous inflow. Inflation of
chamber 2 caused increase at level 3 by 5 mm, of chamber 3 by 5
mm, of chamber 4 by another 3 mm do decrease sharply
(compression by sleeve chamber 4). Inflation of chambers distal to
4 was followed by decrease in girth at level 4 most likely due to an
easy proximal drainage of tissue fluid. The values of girth changes
in mm were used for volume computing. Five mm increase
corresponded to 15-17 ml.
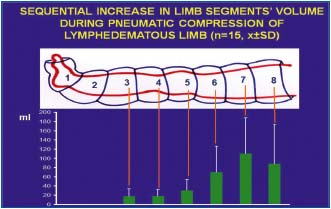
Figure 9. Calculated volumes of tissue fluid (TF) moved
sequentially from distal parts of lymphedematous limb toward the
groin by pneumatic compression of chambers 1 to 7. The volume
increases in consecutive limb segments to reach highest values in
the mid-thigh. Note that although edema is clinically most visible
above the ankle level, bulk of the fluid is moved from the thigh.
Values are means ± SD, n=15. Note high SD caused by differences
in accumulation of TF in individual patients.
Correlation between pressure and girth changes during sequential compression
Correlating pressure and flow data provides information of whether there is a high resistance to tissue fluid flow. High fluid pressures and low flow would suggest major flow obstruction, usually at the groin level, whereas, low pressures and low flows would point to low efficacy of compression due to high skin rigidity. High flows and low pressures were usually seen in early stages of lymphedema without fibrotic changes of skin and subcutaneous tissue. There was no correlation between tissue fluid pressure generated by external compression and flow in proximal direction in tissue fibrosis and scars after surgery and radiotherapy. There could be high pressure but practically no flow . This resistance could only be overcome by strong external compression generating very high tissue fluid pressures.
DISCUSSION AND CONCLUSIONS
Taken together, in obstructive lymphedema of lower limbs tissue fluid pressures in the subcutaneous tissue were low and did not differ from those measured in normal subjects. During manual massage the applied force is set arbitrarily and it generated pressures ranging from 100 to 150 mmHg. Compression at a distance of 3 cm from location of the pressure sensor didn’t not show any rise in pressure what could be explained by skin rigidity and low hydraulic conductivity of subcutis. Tissue fluid flow appeared during manual compression only when the hand was pressing, but stopped immediately after cessation of massage. The calculated flow values during manual massage were low.
Pressures generated in tissue fluid by pneumatic compression chambers were lower than those measured in the inflated chamber. The gradient depended most likely on skin rigidity (fibrosis) and dissipation of the applied force in the subcutaneous tissue to the proximal non-compressed regions. There was little tissue fluid pressure transmission in subcutis to the noncompressed proximal segments. Interestingly, building up pressure in the distal parts of the limb during inflation of proximal chambers was observed. This was presumably the consequence of flow obstruction at the inguinal level. Strain gauge put around the limb provided data on circumference changes during compression and allowed to calculate the approximate volume of displaced fluid. This volume ranged in our studies from 10 to 30 ml per inflated chamber, to reach above 100 per the whole sleeve in the groin region.
Interestingly, there was no direct correlation between tissue fluid pressure generated by external compression and flow in proximal direction. This was presumably caused by fibrosis of the subcutaneous tissue and scars after radiotherapy creating mechanical resistance to flow. It could only be overcome by external compression generating very high tissue fluid pressures.

REFERENCES
1. Bollinger A, Herrig I, Fischer M et al. Intravital capillaroscopy in patients with chronic venous insufficiency and lymphedema: relevance to MPFF at a dose of 500 mg. Int J Microcirc Clin Exp. 1995;15 suppl 1:41-44.
2. Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology. 1996;29:126-131.
3. Cohen SR, Payne DK, Tunkel RS. Lymphedema: strategies for management. Cancer. 2001;15;92 (4 Suppl):980-987.
4. Rockson SG. Lymphedema. Curr Treat Options Cardiovasc Med. 2006;8(2):129- 136.
5. Olszewski WL. The treatment of lymphedema of the extremities with microsurgical lympho-venous anastomoses. Int Angiol. 1988;7:312-321.
6. Brorson H, Ohlin K, Olsson G et al. Controlled compression and liposuction treatment for lower extremity lymphedema. Lymphology. 2008;41:52- 63.
7. Campbell W, Harkin DW. Surgical debulking in a case of chronic lymphoedema. Ir J Med Sci. 2009;178:227-229.
8. Olszewski WL, Jain P, Ambujam G et al. Anatomical distribution of tissue fluid and lymph in soft tissues of lower limbs in obstructive lymphedema—hints for physiotherapy Phlebolymphology. 2009;16:283-289.
9. Olszewski WL. Atlas of Lymphology. Paris, France: Servier; 2001.
10. Olszewski WL, Engeset A. Intrinsic contractility of leg lymphatics in man. Preliminary communication. Lymphology. 1979;12:81-814.
11. Olszewski WL. On the pathomechanism of devolopment of postsurgical lymphedema. Lymphology. 1973;6:35.
