The venous valves of the lower limbs
ABSTRACT
Venous valves appear after the heart begins to beat and the primordial muscles begin to move the limb buds. The pressure gradient due to heart beats and muscle movements triggers the process of venous valve development. Prenatal, postnatal, and senile morphological and numerical rearrangements have been described. However, the signaling pathways related to venous valve morphogenesis have yet to be clearly demonstrated.
The cusps of the venous valves consist of thin collagen half-moon–shaped folds covered by endothelium, which spring from the wall of the vein very close to each other. The vein wall is thicker at the base of the valve cusps, due to an increase in the number of smooth muscle cells in the media. With increasing age, the loose areolar collagen stroma of the cusp is gradually replaced by thick and fibrous tissue.
This article first describes the distribution of the valves in the deep, superficial, and perforating veins of the lower limbs. Finally, the morphology and location of valves in the microveins and their possible role in the pathogenesis of skin changes related to venous insufficiency are described.
1. THE DISCOVERY OF VENOUS VALVES
In his In Anatomen Corporis Humani Tabulae Quatuor published in 1544, Ludovicus Vassaeus was the first to mention the existence of valves within the veins.1 Vesalius did not mention the existence of venous valves (VVs) in the first edition of his De Humanis Corporis Fabrica, but included them after Sylvius Ambianus (1478-1555) described the presence of valves in the veins of the lower limbs. The function of the valves was clearly identified in 1559 by Andrea Cesalpino in his De Re Anatomica: “certain membranes placed at the openings of the vessels prevent the blood from returning.” A few decades later (1585), the German Salomon Alberti published the first drawings of a VV. In 1603, Hyeronymus Fabricius ab Aquapendente (1533-1619) published the first treatise on VVs entitled De Venarum Ostiolis, which included the description of the anatomy and topography of VVs in the whole venous system. More importantly, Fabricius described a test to evaluate VV location and competence (Figure 1) that led his student William Harvey (De Motu Cordis, 1628) to discover the circulation of the blood.2
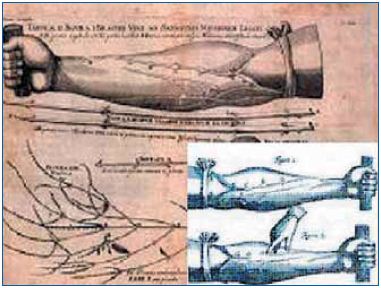
Figure 1. Plate 2 of Fabricius’ De Venarum Ostiolis was
modified by William Harvey (insert) to demonstrate the direction
of flow into the veins.
2. EMBRYOLOGY AND GENETICS OF VENOUS VALVES
Very few studies have investigated the development of VVs in the human embryo. In 1927, Kampmeier and Birch3 described the basic morphogenic events involved in VV development as a five-step process: (i) thickening of the endothelium, which forms a pair of ridges placed transverse to the axis of the vessel; (ii) growth of the endothelial ridges due to their invasion by the underlying mesenchyma, which bulges out of the valvular anlage; (iii) the evolving valve directs itself toward the heart; (iv) The valvular cusps widen into a nodular shape, while the valvular sac gains in capacity; and (v) the venous wall thins down considerably in the region of the valvular sinus, mainly due to thickening of the media (Figure 2).
This pattern of VV development has been confirmed by a recent investigation performed in mice by Bazigou and colleagues.4 The same authors also demonstrated a strong similarity in the morphogenesis and signaling pathways involved in the processes of valve formation in the veins and lymphatics.5 In fact, as well as ephrin-B2 (a key marker of arterial identity), VVs express a repertoire of proteins previously characterized as specific and critical regulators of lymphangiogenesis (Prox1, Vegfr3, and integrin-α9).4 Ephrin-B2 and integrin-α9 signaling is also necessary for the maintenance of VVs.4 Finally, Bazigou and colleagues also found that, at the molecular level, the endothelial cells of VVs closely resemble lymphatic endothelium, which suggests that terminally differentiated endothelial cells exhibit plasticity in their ability to take on a different phenotypic identity.5 Finally, Mellor and colleagues demonstrated the possible role of FoxC2, NFATc1, and VEGFR3 mutations in determining the presence of abnormalities in valve function in great saphenous vein specimens from patients with Milroy’s disease.6,7

Figure 2. The five steps of venous valve development (modified
from reference 4, Bazigou et al, 2011).
VVs only develop after the onset of “a certain pressure gradient along the vein,” as correctly stated in 1926 by Jäger.8 In fact, according to Kampmeier and Birch, the earliest valves of the lower extremity appear “only after the heart begins to beat and the primordial muscles begin to move.” In the lower limbs, VVs appear at approximately 3 to 4 months in the deep veins of the femoral trigone and popliteal fossa and in the upper end of the great saphenous vein.3
Then, VVs increase in number in all areas during prenatal life. Differences in the distribution and characteristics of VVs in different areas of the human body start immediately after birth. In 1981, Maros pointed out that “certain findings suggest a reorganization after birth of the venous valves which are frequently met in fetus (sic). The close relation between hemodynamic mechanisms and the blood guiding structures may explain the changes (disappearance or persistence) of venous valves in some areas after birth.”9
Age-related morphological changes in VV leaflets were comprehensively described by Saphir and Lev in the femoral vein:10 “Starting after the age of 30, the loose areolar collagen stroma of the cusp is gradually replaced by thick and fibrous tissue. After 40, an increase in elastic tissue starts at the base of the cusp, to gradually spar the free margin. In the parietal portion of the leaflet accumulation of dense-collagenous fibers. In the luminal, deposition of collagen between endothelium and elastic membrane.”
Similar changes demonstrating leaflet thickening were described in the great saphenous11 and renal1211,12 and duplex ultrasonography13 confirmed these results. Van Langevelde et al demonstrated the presence of thicker valves in older humans: the mean leaflet thickness was 0.35 mm (range, 0.25 to 0.44 mm) in individuals aged between 20 and 30 years and 0.59 mm (range, 0.30 to 1.21 mm) in individuals aged between 71 and 80 years.13 The increase in valve thickness per year (linear regression coefficient) was 0.004 mm (95% confidence interval, 0 to 0.009). Van Langevelde et al noted that valve function was not directly associated with age but that valve thickness was inversely associated with valve function and that there was a correlation between the age-related increase in valve thickness and the age gradient seen in the incidence of venous thrombosis.13 Interestingly, Chopard and colleagues noted that while the renal vein wall undergoes atrophic changes with increasing age, the corresponding valves show a gradual thickening as a result of an increased number of collagen fiber bundles.12 In conclusion, age related changes in VVs may be attributed to muscular contractions and the physiologic hemodynamic stress related to the standing position rather than significant reflux due to VV dysfunction.
Other authors have postulated that the age-related increase in VV thickness results in a progressive decline in valve function, as demonstrated by the parallel decrease in calf muscle pump efficiency.14 These age related morphologic changes in VVs may also partly explain why aging is such an important risk factor for venous thrombosis.15
These data agree with findings from Marinov, who established that in parallel with aging the number of fully developed valves is reduced while that of “partial” valves is increased, with the process being most pronounced during the period from 25 years to 60 years. In subjects older than 60 years, the number of partial valves represents between 1/5 and 1/10 of the total number of VVs.16
These findings seem to confirm the theory proposed by Bardeleben in 188017 of a systematic and physiologic reduction in the number of VVs in the fetal period and extend it to the whole duration of life. Powell and Lynn also proposed a lifelong progressive reduction in the number of VVs and attributed it to involutive non-inflammatory phenomena.18 This is in contrast with the findings of Leu et al,19 who confirmed in 1979 what Klotz had demonstrated in 1887, ie, that the number of VVs does not decrease with age, but that it is the number of incompetent VVs that increases.20 This was also strengthened by Gottlob and May’s observation that VVs cannot disappear but pathological processes may cause them to become incompetent.21 This can be due to the well-known effects of massive thrombosis, but also to subclinical localized thrombosis of the valvular sinus as described by Sevitt in 1974,22 dilation of the valvular anlage (like in varicose veins), and to other ill-defined regressive phenomena.
In any case, these regressive phenomena are subject to regional differences. According to Gottlob and May, “Venous systems, in which unidirectional flow conditions are prevalent, tend to lose their ‘superfluous’ valves more readily, whereas systems, in which heavier strain is exerted on the valves due to hydrostatic stress or reversed flow, preserve their valves throughout life.”21
3. ANATOMY AND TERMINOLOGY OF THE VENOUS VALVES
According to Saphir and Lev, the cusps of the VVs consist of thin collagen half-moon–shaped folds covered by endothelium, which spring from the wall of the vein very close to each other.10 Their free margins diverge to became attached again at the opposite section of the vein wall. The space between the attachment of the free margins of the cusps is called the commissure (Figure 3). The commissure itself is slightly raised because of a thickening of the vein wall in that area. The cusps are thicker at their bases, where they join the wall of the vein. This thickened attachment of the cusp framework was called “agger” by Franklin,23 and “limbus” or “tuberculum” by others. It is shaped like a double horseshoe with the convex sides arranged distally, and contains smooth muscle cells. The continuations of the free border of the cusp where it meets the vein wall are named “cornua.” The valvular sinus (or pocket) is the space between the cusps and venous wall, which is particularly thin at that level (Figure 4).
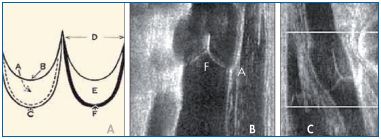
Figure 3. The nomenclature of the venous valves (Panels A and
B) showing the sinus (A), free (B) and attached (C) borders.
D indicates the cornua, E indicates the valve cusps,
and F indicates the agger. Panel C shows that the length of the
cusps is about twice the diameter of the vessel.
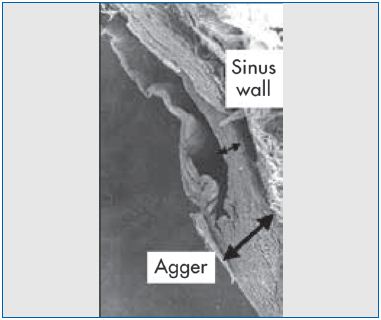
Figure 4. Scanning electron micrograph demonstrating the
difference in wall thickness between the agger and the sinus.
The length of the cusps varies greatly compared with the caliber of the veins. Franklin8 suggested, as a general rule, that the vertical length of the cusps is about twice the diameter of the vessel (Figures 3 and 4).
The cusps can be designated by two faces: luminalis, the part of the cusp close to the lumen of the vein and facing the circulating blood stream, and parietalis, the part of the cusp facing the vein wall of the sinus.
The luminalis is lined by one layer of endothelial cells, which are elongated along the major axis of the vessel. Beneath this layer of endothelial cells is a small amount of connective tissue that is especially noticeable in childhood. Immediately beneath it, there are moderately thick and slightly wavy elastic lamellae, the continuation of the internal elastic lamina. The parietalis is lined by a layer of endothelial cells elongated transversely. The remainder of the parietalis consists of loose connective tissue. At the base of the cusp, the parietalis contains scattered smooth muscle cells extended from the longitudinal muscular bundles of the vein intima. The luminalis and the parietalis join or fuse at the distal end of the cusp, which is thinner than the rest of the cusp. Elastic and connective fibers are thinner there, too. No blood vessels are found within the cusps.
Using scanning electron microscopy of mouse and human VVs, Bazigou4 recently demonstrated morphological differences between the endothelial cells in different parts of the vessel: the cells located upstream of the valve are elongated and aligned in the direction of flow, whereas the cells on the leaflet and downstream of the valve have a rounded morphology, as previously described in venules5 and lymphatic valves. Such differences likely arise from differential exposure to fluid shear stress, which suggests that flow patterns are involved in modulating endothelial cell phenotypes within the valve.
A recent study showed that the valvular sinus endothelium plays an important role in maintaining a thromboresistant state. This is achieved by upregulation of anticoagulant proteins such as the endothelial protein C receptor (EPCR) and thrombomodulin in the valvular sinus endothelium as opposed to the vein lumenal endothelium.24 Von Willebrand factor, a procoagulant protein, is down regulated in the valvular sinus. It may well be that inter-individual or age-related differences in the thromboresistance profile of the valvular sinus endothelium can modulate thrombosis risk.24
The vein wall is thicker at the base of the valve cusps, due to an increase in smooth muscle cells of the media (Figure 4). Part of these cells run circumferentially in bundles, and some run longitudinally and seem to split off from the inner portion of the media to extend into the cusp for a variable extension. Above the agger, the muscular tissue of the vein wall decreases.
Elastic fibers from the internal elastic lamina of the vein extend along the cusp close to the basement membrane of the endothelium. In addition, elastic bundles reinforce the base of the cusps.
Due to its thickness and muscular content, the valvular agger is credited with preventing venous dilation, as first stated in 1603 by Hieronymus Fabricius ab Aquapendente: “quoque venarum distensionem fuisse ostiola a Summo Opifice fabrefacta.” (The Supreme Artificer made valves to prevent venous distension.)
Moreover, the tissue organization of the valve sites suggests that the action of valves is not merely that of passive flaps, but that they can also actively regulate the flow, especially in conditions of low velocity. According to Fegan25 “Contraction of the circular bundles at the base of the cusps reduces the diameter of the vein. Contraction of the longitudinal muscular fibers of the cusps reduces their length and increases their thickness. In addition, the cusps are drawn down towards their roots, and away from each other, but the sphincteric action of the circular bundles compensates for this. The upper free parts of the cusps press against the vein wall at the lateral attachments of the valve, and thus, with the intimal cushions, help to seal this potential leaks…”
4. DISTRIBUTION OF VENOUS VALVES IN THE LOWER LIMBS
Kampmeier and Birch correctly stated that, as a general rule, “Valves are present in those vessels which are subject to pressure from without and in those in the immediate sphere of muscular performance, such as in the veins of the extremities and stomach.”3
Many studies have dealt with the number and location of VVs in the inferior vena cava region.26-29 Data provided by different authors regarding the distribution of valves in the veins of the lower limbs are summarized in the following paragraphs.
The inferior vena cava is without VVs. Sporadic monocuspid valves were exceptionally reported.23 Occasionally, one sporadic and mostly incomplete valve, similar to a spur, is reported in the common iliac vein of 1% to 7% of human limbs.30,31 One VV is in the external iliac vein of about one-fourth of white subjects. In 1882, Friederich noted that one VV is in about 35% of external iliac veins, “…but often mainly decadent.”32 Le Page et al33 reported one well-developed VV located within 2 cm distal to the entrance of the internal iliac vein in 26% of legs and that the external iliac vein has almost three times as many valves as the left (39.6% vs 14.6%). According to Kampmeier and Birch,3 the internal iliac vein is a valvular, whereas its main tributaries (gluteal, sacral, and obturator) are valvular. By contrast, more recently, La Page et al stated that in 7.6% of individuals a well-developed ostial valve is present and that parietal valves are found in only 2.2%.33 Finally, its tributaries are valvular in only 10% of cases.
The common femoral vein shows one VV above the saphenofemoral junction (SFJ), known as the “suprasaphenic valve”.34 It is present in about 70% of limbs35 and protects the saphenous axis against increases in intra-abdominal venous pressure.34 According to Basmajan, two VVs are located in the same tract in about 5% of normal limbs.26 The femoral vein shows about 3 valves. The more constant valve (found in about 90%-100% of cases, according to Banjo31), is located just below the conjunction of the deep femoral vein.36 No data are available with regard to the lateral and medial circumflex veins. The deep femoral vein and the deep femoral perforators are valvular.21 The popliteal veins display between 1 and 3 VVs. Finally, the deep veins of the leg are richly valvular. According to Gottlob and May, 8 to 19 VVs are located in each of the posterior tibial veins, and 8 to 11 VVs are found in both the anterior tibial and peroneal veins.21
Cotton calculated that 7.2±2.3 valves are located along the entire length of the great saphenous vein (GSV).37 According to Raivio, between 1 and 7 valves are located along the thigh portion of the GSV (mean value: 3.5), 2 to 6 are located at the leg (mean value: 4), and, finally, 1 to 4 are located along the marginal veins of the foot.38 The valves of the SFJ are of particular clinical relevance. In 1603, Fabricius stated that the terminal portion of the GSV has a “bicuspid valve at the orifice, then at two fingers’ distance a further another set of twin-valves” (Figure 5). The first is the well-known terminal valve situated at the termination of the GSV to prevent reflux from the femoral vein. The more distal one is the preterminal valve (PTV), which lies just below the openings of the SFJ tributaries. The PTV prevents venous reflux from the SFJ tributaries into the GSV trunk, while the terminal valve is closed. Incompetence of the PTV is the reason for reflux in the GSV in cases in which the terminal valve is still competent.34
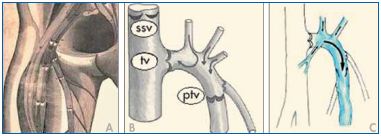
Figure 5. Scanning electron microscopy demonstrating the
difference in wall thickness between the agger and the sinus.
The terminal valve is present in 98% to 99% of normal legs, whereas the PTV is found in only about 70% to 85%. Finally, in about 2% of limbs, no valves are present in the last portion of the GSV.39 According to Raivio, the average number of VVs along the small saphenous vein (SSV) is 8.2.38 The terminal valve is found in about 95% of legs with a saphenopopliteal junction, while the PTV is found in only 64%.40 No data are available on the presence of VVs along the thigh extension of the SSV, which shows, in normal conditions, an antegrade flow directed toward the GSV or toward tributaries of the internal iliac veins (inferior gluteal or ischiatic veins).
Fabricius affirmed that smaller superficial veins (saphenous tributaries, communicating veins, reticular veins) are avalvular. On the contrary, Bouchet41 affirmed that they are valvular at their end, along their course, and at the point of entry of a smaller vein. Duplex ultrasonography easily demonstrates the terminal location of valves in saphenous tributaries (Figures 6-7). Finally, avalvular superficial veins connect the main valvular superficial veins (oscillating veins).42
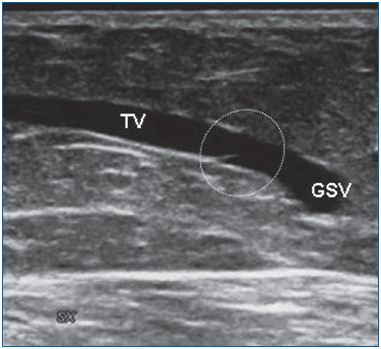
Figure 6. A valve located at the end of a tributary of the great
saphenous vein.
GSV, great saphenous vein; TV, terminal valve.
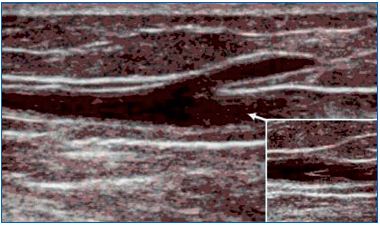
Figure 7. A valve located in the saphenous trunk just below the
opening of the tributary veins.
It is well known that perforating veins are furnished with valves. The number of VVs in perforating valves ranges between 1 and 5 (mean, 2). In 1978, Pirner affirmed that “all valves were found in the subfascial part of the perforating veins.”43 In the same year, Van Limborgh and Hage noted that “the number of valves in the epifascial part of the perforators was significantly less in those (perforating) veins which frequently become incompetent.”44 However, Raivio reported that only 75% of the perforating veins are valvular.38
Avalvular perforating veins are mainly located in the foot, hand, and forearm. However, avalvular perforating veins are reported elsewhere in the human body and work like oscillating veins connecting deep and superficial regions.45,46 This was confirmed by duplex ultrasonography investigations, which demonstrated bidirectional flow in the perforating veins of persons without any sign of venous disease.47-49
While valvular agenesis is a known, but rare, cause of venous insufficiency, the relationship between the number of valves and varicose disease has been poorly investigated. Sales and colleagues demonstrated that the mean number of valves in varicose saphenous veins differed from that of nonvaricose ones (2.3 ± 0.83 vs 4.8 ± 2.01, respectively).50 Banjo comparatively evaluated the presence of VVs in whites and Africans and demonstrated that the number of valves is higher in the latter.31 This may account for the high prevalence (10%-18%) of varicose veins in whites and the low prevalence (1%-2%) of the condition in Africans.
5. VALVES IN MICROVEINS
Anatomists, physiologists, and clinicians still consider the venous bed to be ‘‘valveless’’ from the venular level up to 2-mm veins. On the contrary, microscopic venous valves (MVVs) have been described in the microvascular bed (postcapillary venules and venular efferents of arteriovenous anastomoses), in collecting venules and in small-caliber veins (with diameters up to 800-1000 μM).51
MVVs have been found in the subcutaneous layer and in muscles of various areas of the human body (Table I). Generally, MVVs are described as bicuspid. MVVs are arranged in series along a vein or are situated at the merging point of two veins. The valves always point in the direction of the larger vessel as in collecting veins. Two layers of endothelial cells surround a core of basement membrane material in which bundles of collagen fibrils are embedded.
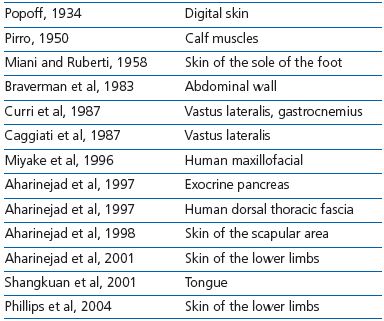
Table I. Presence of microscopic venous valves (MVVs) in tissues
and organs.
MVVs are identical in structure, location, and orientation to the VVs of the leg macroscopic veins. This has led to the hypothesis that their role is to resist blood reflux in small-sized veins and collecting venulae, and to prevent reflux from post capillary venulae to the capillary bed and arteriovenous anastomoses.48 This hypothesis is corroborated by two pieces of evidence: (i) the absence of MVVs in regions with a favorable venous return. (ii) the abundance of MVVs in regions subject to gravitational backflow and where blood flow is irregular or altered by muscular contraction, as with VVs. The absence of MVVs, as well their incompetence, could explain clinical syndromes characterized by signs and symptoms of chronic venous insufficiency in limbs with competent VVs in large veins.52
A recent investigation by Vincent et al has greatly contributed to the knowledge of the anatomy, function, and pathophysiology of microvenous valves in the skin of the lower leg.52 Vincent and colleagues compared findings obtained by scanning electron microscopy of retrograde corrosion casts in legs without detectable venous disease with those of limbs with venous disease, including venous ulcers.
Their study confirmed the existence of valves in microveins of the skin and demonstrated their strategic location along the microvascular tree. Moreover, Vincent and colleagues demonstrated that valvular incompetence at this level can exist independently of valvular competence in the GSV and its accessory tributaries and that tortuosity and distension of varicosities can be found with a normally functioning GSV. They also demonstrated that in the presence of GSV reflux, the competence of valves along the microveins may play a critical role in preventing the progression of the skin changes of venous insufficiency. Finally, they introduced the concept of “boundary valves,” designating those valves located in the more distal veins that appear to prevent reflux in the skin.
These original data strongly support the hypothesis that valve incompetence in both the larger vessel network and the microvenous networks are necessary for skin changes to occur. The authors concluded that the presence/absence and competence/incompetence of valves in the microveins may shed light on several debated clinical questions such as why some patients with very large varicose veins do not experience skin changes.

REFERENCES
1. Caggiati A, Bertocchi P. Regarding “fact and fiction surrounding the discovery of the venous valves”. J Vasc Surg. 2001;33:1317.
2. Caggiati A, Rippa Bonati M, et al. 1603–2003: Four Centuries of Valves. Eur J Vasc Endovasc Surg. 2004;28:439- 441.
3. Kampmeier OF, Birch LF. The origin and development of the venous valves, with particular reference to the saphenous district. Amer J Anat. 1927;38:451-499.
4. Bazigou E, Lyons OTA, Smith A, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984-2992.
5. Bazigou E, Xie S, Chen C, et al. Integrin –alpha 9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;19:175-186.
6. Mellor RH, Brice G, Stanton AW, et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation. 2007;115:1912-1920.
7. Mellor RH, Hubert CE, Stanton AW, et al. Lymphatic dysfunction, not aplasia, underlies Milroy disease. Microcirculation. 2010;17:281-296.
8. Jäger O. Die Entwicklung der Venenklappen. Morph Jb 1926;56:250.
9. Maros T. Data regarding the typology and functional significance of the venous valves. Morphol Embryol. 1981;27:195-214.
10. Shapir O, Lev M. The venous valve in the aged. Am Heart J. 1952;44:843-850.
11. Corcos L, De Anna D. Dini M, et al. Proximal long saphenous vein valves in primary venous insufficiency. J Mal Vasc. 2000;25:27-36.
12. Chopard RP, Miranda Neto MH, et al. Age-related changes in the human renal veins and their valves. Ital J Anat Embryol. 1994;99:91-101.
13. van Langevelde K, Sramek A, Rosenthal FR. The effects of aging on venous valves. Arterioscler Thromb Vasc Boil. 2010;30:2075-2080.
14. Olsen H, Lanne T. Reduced venous compliance in lower limbs of aging humans and its importance for capacitance function. Am J Physiol. 1998;275:878-886.
15. Schina MJ Jr, Neumyer MM, Healy DA, et al. Influence of age on venous physiologic parameters. J Vasc Surg. 1993;18:749-752.
16. Marinov G. Changes with age in the valvular apparatus of the superficial and deep veins of the leg. Eksp Med Morfol. 1973;12:86-91.
17. Bardeleben K. Das Klappen-Distanz- Gesetz. Jenaische Ztschr. Naturwisse. 1880;12:467-529.
18. Powell T, Lynn RB. The valves of the external iliac, femoral and upper third of the popliteal veins. Surg Gynecol Obstet. 1951;92:453-455.
19. Leu HJ, Vogt M, Pfrundes H. Morphological alterations of nonvaricose and varicose veins. Basic Res Cardiol. 1979;74:435-443.
20. Klotz K. Unteresuchungen uber die Vena Saphena Magna bein Menschen. Arch Anat Physiol Anat Abt. 1887;159.
21. Gottlob R, May R. Venous Valves. New York, NY: Springer; 1986.
22. Sevitt S. Organization of valve pocket thrombi and the anomalies of double thrombi and valve cusp involvement. Br J Surg. 1974;61:641-649.
23. Franklin KJ. A monograph on veins. Thomas, Springfield, 1937.
24. Brooks EG, Trotman W, Wadsworth MP, et al. Valves of the deep venous system: an overlooked risk factor. Blood. 2009;114:1276-1279.
25. Fegan WG, Kline L. The course of varicosities in superficial veins of the lower limb. Brit J Surg. 1952;59:798.
26. Basmaijan JV. The distribution of valves in the femoral, external iliac and common iliac veins and their relationship to varicose veins. Surg Gynecol Obstet. 1952;95:537-542.
27. Ludbrook J. Functional aspects of the veins of the leg. Am Heart J 1962;64:706-713.
28. Reagan B, Folse R. Lower limb venous dynamics in normal persons and children of patients with varicose veins. Surg Gynecol Obstet. 1971;132:15.
29. Staubesand J. Kleiner atlas zur vascularen Anatomie der Liestengegend. In: Brunner U, Ed. Die Leiste. Diagnostische und therapeutische Aspekte der Arteriologie, Phlebologie und Lymphologie. Bern, Switzerland: Huber; 1979:12-23.
30. Lindvall N, Lodin A. Congenital absence of valves in the deep veins of the leg. Acta Dermato-Venereologica Scand. 1961;41:45.
31. Banjo AO. Comparative study of the distribution of venous valves in the lower extremities of black Africans and Caucasians: pathogenetic correlates of prevalence of primary varicose veins in the two races. Anat Rec. 1987;217:407- 412.
32. Friederick N. Ueber das verhalten der klappen in den crural-venen. Morphol Jahrb 1882;7:323-325.
33. La Page PA, Villavicencio JL, Gomez ER, et al. The valvular anatomy of the iliac venous system and its clinical implications. J Vasc Surg. 1991;14:678- 683.
34. Caggiati A, Bergan JJ, Gloviczki P, et al. Nomenclature of the veins of the lower limbs: Extensions, refinements and clinical application. J Vasc Surg. 2005;41:719-724.
35. Mühlberger D, Morandini L, Brenner E. An anatomical study of femoral vein valves near the saphenofemoral junction. J Vasc Surg. 2008;48:994-999.
36. Moore HM, Gohel M, Davies AH. Number and location of venous valves within the popliteal and femoral veins: a review of the literature. J Anat. 2011;219:439-443.
37. Cotton LT. Varicose veins: gross anatomy and development. Br J Surg. 1961;48:589-598.
38. Raivio E. Untersuchungen über die Venen der unteren Extremitaten. Ann Med Exp Fenn. 1948;26(suppl 4):213- 216.
39. Hesse E, Schaack W. Die Klappenverhaltnisse der Oberschenkelvene und der Vena Saphena Magna. Vircow’s Archiv. 1911;205:145-154.
40. Schweighofer G, Mühlberger D, Brenner E. The anatomy of the small saphenous vein: fascial and neural relations, saphenofemoral junction, and valves. J Vasc Surg 2010 51:982- 989.
41. Bouchet A. Anatomie morphologique des valvules des membres inferieurs. Phlebologie. 1992;45:233-245.
42. Taylor GI, Caddy CM, Watterson PA, et al. The venous territories (venosomes) of the human body: experimental study and clinical implications. Plast Reconstr Surg. 1990;86:185-213.
43. Pirner F. On the valves of the perforating veins. In: May R, Partsch H, Staubesand J, eds. Perforating Veins. Munich, Germany: Urban and Schwarzenberg; 1979:208-213.
44. Van Limborgh J, Hage RW. Anatomical features of those perforating veins of the leg which frequently or infrequently become incompetent. In: May R, Partsch H, Staubesand J, eds. Perforating Veins. Munich, Germany: Urban and Schwarzenberg; 1979:49- 59.
45. Barber RF, Shatara FI. The varicose disease. NY State J Med. 1925;25:162- 166.
46. Hadfield JIH. The anatomy of the perforating veins in the leg. In: The treatment of varicose veins by injection and compression. Stoke Mandeville Symposium. 1971:4-11.
47. Sarin S, Scurr JH, Smith PD. Medial calf perforators in venous disease: the significance of outward flow. J Vasc Surg. 1992;16:40-46.
48. Stuart WP, Adam DJ, Allan PL, Ruckley CV, Bradbury AW. The relationship between the number, competence, and diameter of medial calf perforating veins and the clinical status in healthy subjects and patients with lower-limb venous disease. J Vasc Surg. 2000;32:138-143.
49. van Neer PA, Veraart JC, Neumann HC Venae perforantes: a clinical review. Dermatol Surg. 2003;9:931-942.
50. Sales CM, Rosenthal D, Petrillo KA, et al. The valvular apparatus in venous insufficiency: a problem of quantity? Ann Vasc Surg. 1998;12:153-155.
51. Caggiati A, Phillips M, Lametschwandtner A, et al. Valves in small veins and venules. Eur J Vasc Endovasc Surg. 2006;32:447-452.
52. Vincent JR, Jones GT, Hill GB, van Rij AM. Failure of microvenous valves in small superficial veins is a key to the skin changes of venous insufficiency. J Vasc Surg. 2011;54:62-69.
