The transosseous perforator veins of the knee
Marios VALSAMIS,2 MD;
Claude GILLOT,3 MD
1Research Director, UNESCO Chair of
Digital Anatomy – Paris University, Paris,
France
2Phlebologist & Surgeon (private practice),
Athens, Greece
3Laboratory of Anatomy – Paris University,
Paris, France
Abstract
The perforator veins (PVs) of the knee can be responsible for reticular veins, telangiectases, and varicosities around the knee, but they are frequently underdiagnosed and missed by the phlebologists and the sonographers because of their tiny size. These PVs are frequently located around the patella and connected with transosseous perforators, well-demonstrated in the anatomical part of this work. This could explain why sclerotherapy of cosmetic lesions around the knee frequently leads to poor results and recurrence. This article proposes a systematization of these atypical PVs and discusses their possible role in phleboarthrosis.
Introduction
The anatomical part of this work constitutes the last work carried out with Professor Claude Gillot who passed away in his 92nd year. Thus, in a way, this article is a tribute to his talent as an anatomist, his sagacity as a researcher, and his outstanding qualities as a teacher.
In fact, while studying anatomical sections that he had prepared more than 20 years ago, he discovered the bone perforators of the knee. “In anatomy, the most difficult task is to see what is happening below your own eyes,” he liked to repeat. It was through noting the presence of green latex inside the spongy bone of the knee that Claude Gillot discovered these tiny perforating veins that perforate the cortex of the tibia and the femur.
Background
In the knee, the spongy bone of the tibial and femoral epiphyses is an important source of production of red blood cells, even more so than the lumbar spine. The red cells produced there go on to join the venous network. They do so via the femoropopliteal venous axis through small veins that perforate the cortical bone. The deep, unusual location of these veins and their tiny size explain why they are often unrecognized and missed by the sonographers during color duplex investigations.
Materials and methods
This anatomical study is based on 40 series of layered anatomical sections of the lower limbs prepared 20 years ago. These are axial sections of the lower limbs taken every 0.5 cm, work carried out in the anatomy laboratory of the Faculty of Medicine at Descartes University.
Before sections were taken, the venous system of the limb was washed and injected with latex preparations. Veins were injected with green latex and arteries with red latex. More information about this technique is available in the Atlas of Venous Anatomy.1
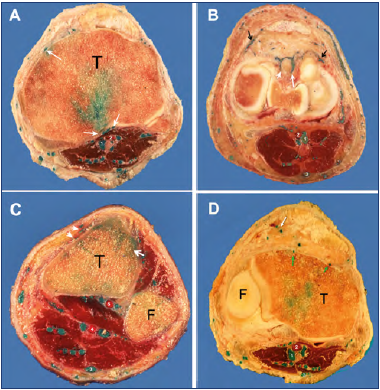
Figure 1. Transosseous perforator veins (PVs) of the knee. Axial sections obtained after latex injection of the limb. Sections were made at the level of the tibial tuberosity (A,) lower condyles (B), and upper leg just below the knee joint (C-D).
A) The green latex has reached the spongious bone. Posterior transosseous PVs perforate the cortical line of the tibia (white arrows).
B) Transfascial PVs are visible anteriorly, indicated by black arrows. Additional trans-synovial perforators are visible, passing between the tibial condyles (white arrows).
C) At the level of the tibial tuberosity, the anterior transosseous PVs (white arrows) drain into the cancellous bone.
D) Small transfascial PVs are visible anteriorly, lateral to the rotulian tendon (white arrow); a small TOPK is shown with a hole in the cortical bone of the tibial tuberosity (green arrows).
Abbreviations: 1, popliteal vein (green circle); 2, popliteal artery (red circle); 3, small saphenous vein; 4, posterior tibial artery surrounded by its two veins; 5, anterior tibial artery surrounded by its two veins; T, tibia bone; F, fibula.
From the collection of Professor C. Gillot.
In addition to the study of those sections, color duplex investigation was carried out before treatment in about 25 000 patients with varicose veins. Preoperative investigation was carried out via computed tomographic (CT)-venography2-4 in about 1200 venous patients and magnetic resonance (MR) angiography in a few.
Results of our anatomical study
According to our anatomical documents,5 the transosseous PVs of the knee (TOPKs) can be categorized into two subgroups: (i) anterior, around the patella (Figure 1 A-C); and (ii) posterior, in the condylian groove and deep popliteal fossa (Figure 2).
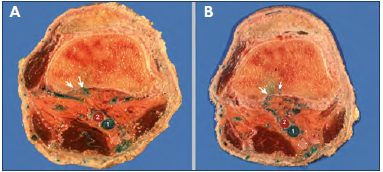
Figure 2. Transosseous perforator veins (PVs) of the high popliteal fossa.
Axial anatomical sections of the lower femur obtained above the knee joint after latex injection of the limb.
The white arrows show the holes of the cortical bone and the posterior perforators joining the spongy bone. They gather at the popliteal fossa, then bypass the popliteal artery (2) to connect the popliteal vein (1). No perforator is seen at the anterior aspect of the knee.
From the collection of Professor C. Gillot.
The first connects to the peripatellar superficial reticular venous network and to the lateral faces of the knee. The second group of perforators crosses the intercondylian space and the deep popliteal fossa bypassing the two sides of the popliteal artery to join the popliteal vein.
Clinical presentations
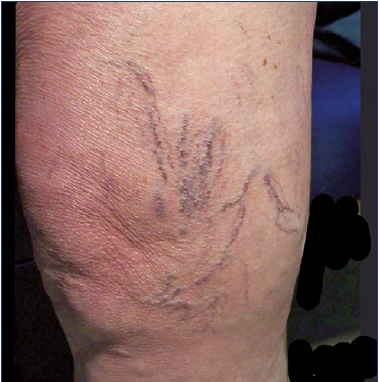
Reticular venous networks and varicose veins around the knee are quite common in clinical practice. They are usually located anteriorly, around and below the patella (Figures 3-4), connected with a varicose network of the anteromedial and lateral aspects of the upper leg.
Telangiectases of the medial or lateral aspect of the knee and lower thigh are also very common in chronic venous disease (CVD) patients (Figure 5).
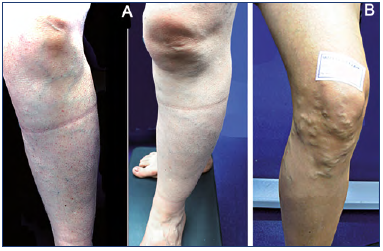
Figure 3. Clinical presentations.
A) Clinical case 1: reticular vein network of the anterolateral aspect of the knee and upper leg.
B) Clinical case 2: varicose vein network of the anterior knee below the patella.
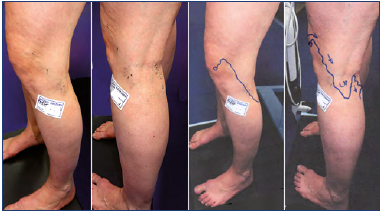
Figure 4. Clinical case 3: popliteal varix fed by a suprapatellar perforator vein.
Skin mapping is shown on the right.
Color duplex assessment
The PVs can be seen on ultrasound surrounding the patella. They are difficult to detect because of their small caliber. They are connected to the superficial reticular network, then perforate the fascia and the aponeurosis around the patella (Figures 6-8). Although no direct image of their transosseous connection is seen in the case reports, we know such PVs exist within the anterior tuberosity of the tibia (Figure 1A,C) and within the intercondylar groove (Figure 1B).
CT-venography
CT-venography with 3D reconstruction by volumetric rendering technique (VRT) is useful for obtaining a complete 3D map of the venous network. The TOPKs are especially visible around the patella (Figure 9).
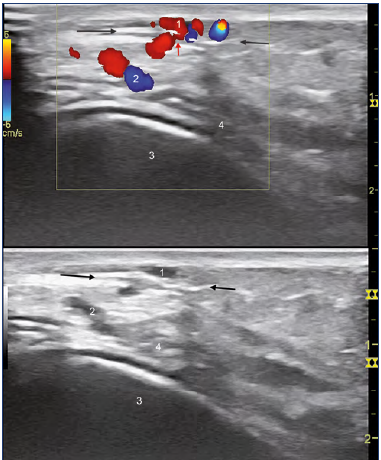
Figure 6. Color duplex assessment of case 2.
The superficial reflux (1) is fed by a perforator vein (PV, red arrow) perforating the fascia (black arrows).
The subfascial refluxing network (2) is close to the bone periosteum (4,) not injected here.
1, superficial network refluxing; 2, subfascial veins; 3, bone.
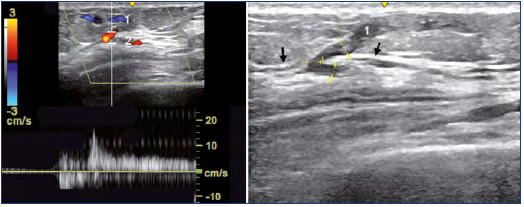
Figure 7. Color duplex assessment of case 3.
Perforator vein (PV) located at the lateral aspect of the patella.
The superficial reflux (1) is fed by a PV (red colored) perforating the fascia (black arrows). The subfascial refluxing network (2).
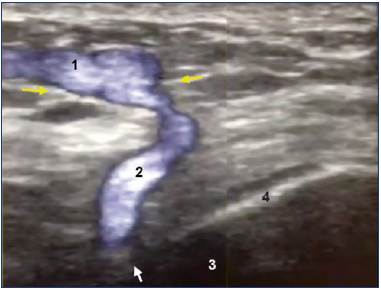
Figure 8. Color duplex assessment of a tibial transosseous perforator vein of the knee.
Ultrasound showing a transosseous perforating vein of the anterior knee. The superficial reflux (1) is fed by a PV (2) perforating the fascia (yellow arrows) and then the cortical bone (4) at the level of the white arrow.
3, tibia bone.
Image courtesy of V. Crebassa, MD.
MR-Angiography
MR angiography investigation is a good method to obtain more anatomical details about the posterior TOPK and around the patella (Figures 10-11). It is also useful for investigating knee arthrosis, frequently associated with severe chronic venous insufficiency (CVI), which will be touched on later in the Discussion.
In many cases, TOPK are an excellent explanation for the difficulty with telangiectasia sclerosis of the knee region and their high recurrence rate. Thus, they should be carefully considered when targeting treatment, as they are often the source of reflux responsible for telangiectasias and reticular veins around the knee.
Differential diagnosis
These TOPK must be distinguished from the rare and large transosseous perforators of the tibial diaphysis, which can feed anterior varicose veins of the leg, described by Ramelet et al6 (Figure 12). These are located in the middle part of the tibia; note the bony hole of the perforator visible on a standard x-ray of the tibia, as well as by duplex ultrasound investigation.
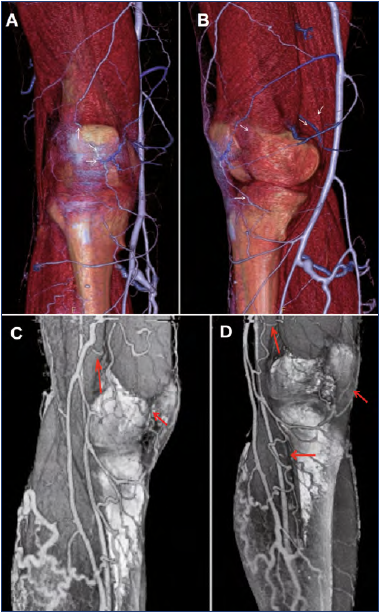
Figure 9. Transosseous perforator veins of the knee surrounding the knee. Three-dimensional reconstruction from veno–computed tomography (CT) by volume rendering (VRT).
A,B) The white arrows show small perforator veins of the knee surrounding the patella connected to the tributaries of the great saphenous vein.
C,D) The red arrows show small tributaries around the knee, mainly at the medial aspect. Courtesy of Professor J. Ovelar and J. Merino – CIMED – La Plata, Argentina.
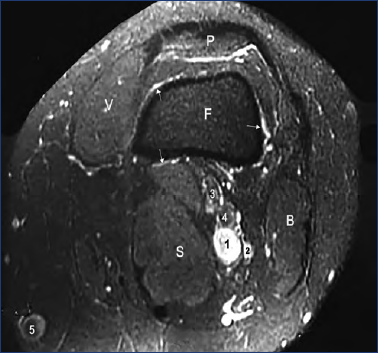
Figure 10. Axial angioMR, slice at the patella level.
No PV is shown through the cortical bone, but we see a venous network surrounding the bone (arrows), connected to the superficial network.
1, popliteal vein; 2, collateral canal; 3, tibial nerve; 4, fibular nerve; 5, great saphenous vein; F, femur; P, patella; V, vastus medialis; B, biceps; S, semimembranosus.
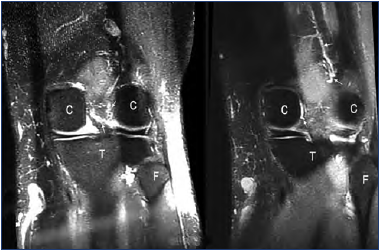
Figure 11. Coronal angioMR: slice taken at the condyles level.
Venous network located in the intercondylar groove (arrows).
Abbreviations: C, femoral condyles, T, tibia; F, fibula head.
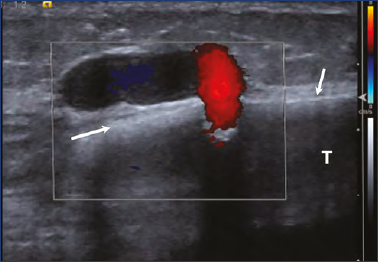
Figure 12. PV of the tibial diaphysis (Color Duplex).
Arrows indicate cortical bone.
Abbreviation: T, tibia bone.
Discussion
The close relationship between severe CVI and knee arthrosis was emphasized many years ago by several German authors7-19 and described as “Das arthrogene Stauungssyndrom” and called “phlebo-arthrosic syndrome” or “phleboarthrosis.” These features could also be linked to regional dermato-lipo-fasciosclerosis observed in the framework of severe CVI. Conversely, gonarthrosis20 is a cause of impairment of joint mobility and thus of the venomuscular pumps, worsening CVI.
The case-control study of Mazieres21 reported an 18% to 64% prevalence of varicose veins in gonarthrosis patients and a 12% to 68% prevalence of gonarthrosis in varicose vein patients. The simultaneous existence of such arthrosis and varices in the same lower limb has also been reported in 48%, by Gies,22 and 20% by Robecchi.12
More evidence of the role of venous stasis in lower limb arthrosis is brought by several case reports of improvement of the inflammatory component of osteoarthritis of the knee,18-20,23-38 hip,39,40 or ankle after treatment of varicose veins. The recent study by Varghese et al41 also brings new perspective to the understanding of the pathophysiology of osteoarthritis, for which venous stasis is an underlying cause.
Clinical diagnosis of phleboarthrosis is the most important aspect in planning the treatment protocol. Once the venous reflux is treated, knee joint inflammation shifts toward normality. Pain decreases noticeably and arthrosis begins to calm. The orthopedic surgeon can enhance this healing by conservative measures. In this study, over a period of 6 months, all patients improved and were able to resume normal activities despite long-term history of osteoarthritis.
We hypothesize that the TOPK play an important role in this “phlebo-arthrosic syndrome” by transmitting venous hypertension to the knee joint structures. So, general practitioners and phlebologists should take into account the role played by CVI in the genesis of knee osteoarthrosis: these small TOPKs probably play a role in the “phleboarthrosis” described in the literature. In fact, poor nourishment of the bone marrow and every structure around the knee joint occurs when there is venous hypertension and stasis related to severe CVI. Conversely, osteoarthritis of the knee or ankle limits movement and impedes walking, and thus could be responsible for impairment of venous pumps, worsening CVI. In practice, more practitioners should be aware of this frequent cause of osteoarthritis.
In patients suffering from disabling knee arthritis associated with severe venous insufficiency of varicose origin, the varicose veins should be treated first, before proposing surgery of the knee. In most cases, the inflammatory component of the knee arthritis will improve significantly, and the surgery can be postponed.
Conclusion
Phlebologists should be aware of the existence of PVs of the knee, mainly around the patella, in order to target sclerotherapy of telangiectasias and reticular veins under echoguidance.
They should also keep in mind that these subfascial PVs are frequently fed by transosseous PVs, even if they cannot be seen easily with duplex color imaging.
Echoguided elective treatment of the knee PVs by foam sclerotherapy works well. This should improve cosmetic results and avoid recurrence, too frequent in this area.
Moreover, regarding phleboarthrosis, it’s important that phlebologists and rheumatologists be aware that severe CVI is a common cause of osteoarthritis of the knee and ankle and that they should, therefore, be treated in priority, before the treatment of arthrosis.

REFERENCES
1. Uhl JF. Atlas anatomique du système veineux. 2020. Book available from Uhl JF: jeanfrancois.uhl@gmail.com.
2. Uhl JF. Three-dimensional modeling of the venous system by direct multislice helical CT venography: technique, indications and results. Phlebology. 2012;27:270-288.
3. Uhl JF, Caggiati A. Three-dimensional evaluation of the venous system in varicose limbs by multidetector spiral CT. In: Catalano C, Pasariello R, eds. Multidetector-Row CT Angiography. Medical Radiology (Diagnostic Imaging). Springer, Berlin, Heidelberg. Available at: https://doi.org/10.1007/3-540-26984- 3_15.
4. Uhl JF, Verdeille S, Martin-Bouyer Y. Threedimensional spiral CT venography for the pre-operative assessment of varicose patients. VASA. 2003;32:91-94.
5. Gillot C, Uhl JF, Ovelar J, Merino J. Anatomy of the bony perforators veins of the knee. Ann Med. 2019:51(suppl 1):60- 60. doi:10.1080/07853890.2018.156194 3.
6. Ramelet AA, Crebassa V, Alotto CD, et al. Anomalous intraosseous venous drainage: bone perforators? Phlebology. 2016;32(4);241-248.
7. Adler VG. Diagnosis and treatment of phlebo-arthrotic syndrome. Med Klin. 1956;51(34):1407-1409.
8. Annas T. The phlebo-arthrotic syndrome in orthopedics [in German]. Med Welt. 1980;31(14):523-524.
9. Hach W, Langer C. Schirmers u das arthrogene stauungssyndrom. VASA. 1983;12:109-115.
10. Hach W. Das arthrogene stauungssyndrom. Geßässchirurgie. 2003;8:227-233.
11. Hach W. VenenChirurgie. 2nd ed. Schattauer, Germany: Stuttgart; 2006;304- 306.
12. Robecchi A, Einaudi A, Impallomeni G. Osservazioni e ricerche sui fattori eziopatogenetici dell’artrosi del ginocchio, con particolare riguardo ai disturbi della circolazione venosa. Minerva Med. 1952;43(101):1366-1376.
13. Krieg E. The phlebo-arthrotic complex. Med Welt. 1970;42:1832-1834.
14. Giovanni B, Agus MA. Phleboarthrosis. Acta Phlebologica. 2017;18(3):63-64.
15. Schmeller W. Das Arthrogene Stauungssyndrom. Sprunggelenksveränderungen bei Chronischer Veneninsuffizienz. Berlin, Germany: Diesbach Verlag; 1990.
16. Noppeney T, Nüllen H. Arthrogenes stauungssyndrom. In: Noppeney T, Nüllen H, eds. Diagnostik und Therapie der Varikose. Heidelberg, Germany: Springer Medizin Verlag; 2010;S 213-214.
17. Frendel A, Hamper C, Kahle B, et al. Das arthrogene stauungsssyndrom. Phlebologie. 2015;44:215-217.
18. Arnoldi CC. Vascular aspects of degenerative joint disorders a synthesis. Acta Orthop Scand. 1994;65(suppl 261):3-82. doi:10.3109/17453679409155226.
19. Marshall M. Praktische Phlebologie “Phlebarthrotischer Symptomenkomplex.” Springer-Verlag; 1987:81. doi:10.1007/978-3-642-70468.
20. Kugler C, Strunk M, Rudofsky G. Effect of impaired joint mobility on venous pump function of the healthy lower limb, a phlebodynamometric analysis. Phlebologie. 1999;28(1):16-22.
21. Mazieres B, Andrieu S, Subreville C, et al. Knee osteoarthritis and varicose veins: a case-control study of 600 patients. Ann Rheum Dis. 2001;60:A175-A176.
22. Gies J, Maugeis De Bourguesdon J. Value of the presentday treatments of gonarthrosis [in French]. Rev Rhum Mal Osteoartic. 1961;28:255-258.
23. Imhof H, Breitenseher M, Kainberger F, Trattnig S. Degenerative joint disease: cartilage or vascular disease? Skeletal Radiol. 1997;26(7):398-403. doi:10.1007/ s002560050254. PMID: 9259096.
24. Lejoyeux R. Study of a case of phleboarthrosis. Treatment with strapping and sclerosing injections [in French]. Phlebologie. 1972;25(1):35-37.
25. Doerler M, Altmeyer P, Stücker M. Venous leg ulcer caused by obesity-associated dependency syndrome Case report and discussion of the pathogenesis and treatment. Phlebologie. 2013;42:205-208.
26. Lentner A. Beweglichkeit im oberen und unteren Sprunggelenk bei fortgeschrittener chronischer veneninsuffizienz. Phlebologie. 1994;23:149-155.
27. Ludwig M, Rieger J, Ruppert V. Gefäßmedizin in Klinik und Praxis – Leitlinienorientierte Angiologie, Gefäßchirurgie und interventionelle Radiologie. 2nd edition. Stuttgart, Germany: Thieme; 2010:283-284.
28. Uhl JF, Chakim M, Allaert FA. Static foot disorders: a major risk factor for chronic venous disease? Phlebology. 2012;27:13-18.
29. Lesnyak OM, Zubareva EV, Goncharova MG, Maksimov DM. Lower extremity venous diseases in primary knee osteoarthritis [in Russian]. Ter Arkh. 2017;89(5):53.
30. Arnoldi CC, Lemperg K, Linderholm H. Intraosseous hypertension and pain in the knee. J Bone Joint Surg Br. 1975;57(3):360-363.
31. Rabe E, Berboth G, Pannier F. Epidemiology of chronic venous diseases [in German]. Wien Med Wochenschr. 2016;166(9-10):260-263. doi:10.1007/ s10354-016-0465-y.
32. Helal B. The pain in primary osteoarthritis of the knee. Its causes and treatment by osteotomy. Postgrad Med J. 1965;41(474):172-181. doi:10.1136/ pgmj.41.474.172.
33. Dodd H, Cockett FB. The Pathology and Surgery of Veins of the Lower Limb. London, UK. E. & S. Livingstone; 1956.
34. Ciubotaru V. Phlebogonarthrosis: a clinical and physiopathological reality. Phlebolymphology. 2018;25(1):1-120.
35. Findlay DM. Vascular pathology and osteoarthritis. Rheumatology. 2007;46(12):1763-1768. https://doi. org/10.1093/rheumatology/kem191.
36. Bernstein MA. Experimental production of arthritis by artificially produced passive congestion. J Bone Jt Surg. 1933;15:661.
37. Ageeva AI, Kulikov AG, Volovets SA, Gerasimenko MY, Yarustovskahya OV. Gonarthrosis concurrent with chronic venous insufficiency: a new look at therapy [in Russian]. Vopr Kurortol Fizioter Lech Fiz Kult. 2019;96(5):29.
38. Reinharez D. Effect of chronic venous insufficiency in gonarthrosis [in French]. Phlebologie. 1981;34(1):187.
39. Pedersen NW, Kiaer T, Kristensen KD, Starklint H. Intraosseous pressure, oxygenation, and histology in arthrosis and osteonecrosis of the hip. Acta Orthop Scand. 1989;60(4):415-417. doi:10.3109/17453678909149309.
40. Arnoldi CC, Linderholm H, Müssbichler H. Venous engorgement and intraosseous hypertension in osteoarthritis of the hip. J Bone Joint Surg Br. 1972;54(3):409-421.
41. Varghese R, Patel M, Rajarshi M. Association of chronic venous disease and arthrosis of the lower limbs. Clinic-based Indian experience of phleboarthrosis and treatment protocol. Circulation. 2021. In press.