Protection of the microcirculation during ischemia/reperfusion isenhanced by micronization of purified flavonoid fraction
D. A. BOTTINO,
E. BOUSKELA
em Microcirculação,
Universidade do Estado do Rio de Janeiro,
Rio de Janeiro, Brazil
ABSTRACT
This study was designed to evaluate the importance of micronization on the protective effect of micronized purified flavonoid fraction (MPFF) on increases in macromolecular permeability induced by ischemia/reperfusion in the hamster cheek pouch microcirculation. Male hamsters (Mesocricetus auratus) were treated orally, twice a day, with vehicle (lactose), MPFF and nonmicronized purified flavonoid fraction (PFF) at 5, 20, 80, and 320 mg/kg per day for 10 consecutive days. On the 11th day, cheek pouches of anesthetized animals were prepared for intravital microscopy. Local ischemia of 30 min was obtained by clamping the neck of the everted pouch and the increase in microvascular permeability was quantified as leakage (leaks) of intravenously injected fluorescein isothiocyanate-labeled dextran. Reperfusion resulted in an immediate but reversible increase in postcapillary leakage. MPFF induced a significant dose-dependent reduction in the increased permeability (control: 115.7 ± 4.1 and 320 mg/kg body weight/day: 19.2 ± 1.9 leaks/cm2, P<0.0001). Nonmicronized PFF was significantly less effective (60.3 ± 1.0 leaks/cm2, also for the highest dose, P<0.0001). In conclusion, micronization significantly enhances the protective effects of MPFF, and this improvement is probably related to the better absorption of the micronized formulation and could explain the superior clinical efficacy shown in previous studies.
INTRODUCTION
Micronized purified flavonoid fraction (MPFF) contains 90% of micronized diosmin (diosmetin-7-rhamnoglucoside) and 10% flavonoids expressed as hesperidin (hesperitin-7-rhamnoglucoside). Clinically it is used to treat chronic venous insufficiency and hemorrhoidal disease, due to its anti- inflammatory properties.1,2 The pathophysiology of chronic venous insufficiency seems to involve cyclic periods of ischemia/reperfusion.3,4 Early reperfusion of the ischemic tissue is essential to stop the progression of cellular injury associated with oxygen and nutrient depletion. However, it has already been demonstrated that reperfusion to an ischemic area produces a complex cascade of pathological events which could potentially lead to the same end result as prolonged hypoxia, which is cellular dysfunction and necrosis. Reactive oxygen species induce the production of proinflammatory agents like platelet-activating factor, leukotriene B4, and activated complement components. They also modify the expression of adhesion molecules on the surface of leukocytes and endothelial cells (CD11b/CD18, P-selectin and intercellular adhesion molecule-1) and reduce the levels of nitric oxide (NO) due to decrease in NO-synthase in the muscle, NO-synthase being a potent vasodilator and antiadhesive substance.5 The activated leukocytes migrate into the interstitial tissues, inducing microvascular barrier dysfunction via release of oxidants and hydrolytic enzymes.6 Flavonoids inhibit the phosphodiesterases involved in cell activation. This effect is mainly produced by the biosynthesis of cytokines that mediate adhesion of circulating leukocytes to sites of injury. Thus, enhanced vascular macromolecular leakage is one of the earliest signs of microvascular dysfunction elicited by ischemia/reperfusion. The increased permeability leads to interstitial edema, which will lead in time to physical compression of capillaries promoting the development of the no-reflow phenomenon.7,8
Micronized purified flavonoid fraction (MPFF) has been shown to exert a protective effect on the microvascular barrier function in experimental conditions, such as ischemia/reperfusion,9-11 oxidative stress,12 inflammation, 9,13 or venous hypertension.14
This study was designed to evaluate the influence of micronization on the protective effects of the purified flavonoid fraction on microvascular barrier disruption.
The dose-related effects of the micronized form were compared to those of the nonmicronized form on macromolecular permeability increase induced by ischemia/reperfusion using the hamster cheek pouch.15
MATERIAL AND METHODS
Animals
Male golden hamsters (Mesocricetus auratus), 7 to 10 weeks old, weighing approximately 100 g, were obtained from Engle Labs., Farmersburg, Indianapolis, USA. The experiments were performed according to protocols approved by the Ethical Committee of the State University of Rio de Janeiro (H36/94). The animals received an appropriate laboratory diet, Nuvital, from Nuvilab, PR, Brazil.
Experimental Groups
There were nine animal groups (n=6, each), MPFF and nonmicronized purified flavonoid fraction (PFF) at different doses and with different vehicles. The drugs were obtained from Servier Laboratories (Gidy, France) and were suspended in 10% lactose solution, before each administration. The vehicle, 10% lactose solution, MPFF and nonmicronized PFF, at doses of 5, 20, 80, and 320 mg/kg body weight/day, were administered by gavage, twice a day, at 8:00 AM and 5:00 PM, for 10 consecutive days. Each animal received 0.2 mL of suspension per 100 g body weight. The investigator was blinded for formulation being administered (micronized or nonmicronized), in order to avoid bias. The last dose was given 30 min before the induction of anesthesia.
Surgical Procedures
Intraperitoneal injection of sodium pentobarbital (Pentobarbital sodique, Sanofi, Paris, France, 60 mg/mL) was used to induce anesthesia, maintained with _-chloralose [1,2-O-(2,2,2-trichloethylidene) _-D-glucofuranose], (Merck, Darmstadt, Germany, 100 mg/kg) administered through the femoral vein. The femoral artery was cannulated to measure arterial pressure. During the experiment, the temperature of the animals was maintained at 37.5° C with a heating pad controlled by a rectal thermistor. A tracheal tube was inserted to facilitate spontaneous breathing. The hamster was placed on a microscope stage similar to that described by Duling15 and Svensjö and coworkers,16 modified by Bouskela and Grampp.17 The cheek pouch was everted and pinned with four to five needles into a circular well, filled with silicone rubber to provide a flat, bottom layer, thus avoiding stretching of the tissue, but preventing shrinkage. In order to produce a single-layer preparation, an incision was made in the upper layer so that a triangular flap could be displaced to one side. The exposed area was dissected at 10-16X magnification under the stereomicroscope, and the fibrous, almost avascular, connective tissue covering the vessels was removed using ophthalmic instruments. The dissected part of the pouch was 125 to 150 μm thick. Pouches with petechial hemorrhages or without blood flow in all parts were discarded.
The superfusion solution was a HEPES-supported HCO-3-buffered saline solution; the temperature was maintained at 36.5° C and the superfusion rate was 4 mL/min. The pH was set to 7.40 by bubbling the solutions continuously with 5% CO2 in 95% N2.
Thirty minutes after the completion of the preparative procedure, FITC-dextran 150 (Bioflor HB, Uppsala, Sweden) was given at a dose of 25 mg/100 g as an intravenous injection of a 5% solution in 0.9% saline.16 Observations were made with a Leitz Ortholux microscope with a x3.5 objective and x10 oculars.
Local ischemia of the cheek pouch was produced with a cuff, made of thin latex tubing, which was mounted around the neck of the everted pouch where it leaves the mouth of the hamster.18 The intracuff pressure could be rapidly increased by air compression by using a syringe and could be just as rapidly decreased when required. An intracuff pressure of 200-220 mm Hg resulted in a complete arrest of the microvascular blood flow within a few seconds (Figure 1).
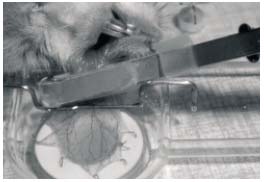
Figure 1. Local ischemia of the cheek pouch. A latex cuff is mounted
around the neck of the everted pouch where it leaves the mouth
of the hamster.
The fluorescent spots seen at leakage sites could be counted when they reached at least 100 μm size.9 The leakage was expressed per cm2. The number of leaks was obtained before, immediately after the ischemic period, and every 10 min thereafter for 1 hour. All hamsters with the prepared area showing spontaneous nonfading leaks or more than 10 fading leaks during the first 30-minute control period, after FITC-dextran was given, were discarded.
The results are presented as mean ± SEM. After a oneway analysis of variance, comparison of each dose of MPFF and nonmicronized PFF with the vehicle-treated group was evaluated using the bilateral Dunnett’s test. Comparisons between two groups were performed by contrast and Bonferroni’s method was used to compensate for multiple tests.
RESULTS
No significant difference in mean arterial pressure could be detected among the groups treated with vehicle or any of the treatment formulations.
Figure 2 shows an example of microvascular leakage. The fluorescent spots indicating edema are located in the microscope field, mainly at the right side.
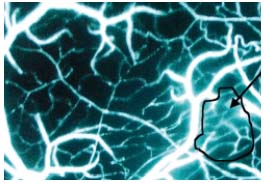
Figure 2. Example of microvascular leakage during reperfusion
phase. Black arrow shows white fluorescent spots of FITC-dextran
indicating edema.
The maximal numbers of venular leakage sites per cm2 during reperfusion, after 30 min of total ischemia, in animals treated for 10 days with either vehicle, MPFF, or nonmicronized PFF can be seen in Figure 3. Reperfusion resulted in a reversible increase in postcapillary leakage (leaks). In vehicle-treated hamsters, the mean maximal response was 115.7 ± 4.1 leaks/cm2. Oral treatment with MPFF inhibited, in a dose-dependent fashion, the macromolecular permeability increase induced by ischemia/ reperfusion. At the highest dose, 320 mg/kg body weight/day, there was a 83.4% inhibition (19.2 ± 1.9 leaks/cm2, P<0.0001). Oral treatment with the nonmicronized PFF also induced a significant reduction in macromolecular permeability increase induced by ischemia/reperfusion compared with the vehicle-treated group, but to a lesser extent and without a dose-dependent relationship. The maximum decrease in plasma leakage with nonmicronized PFF was obtained at the dose of 80 mg/kg body weight/day, with a 48.8% inhibition (59.3 ± 1.7 leaks/cm2, P<0.0001). For each dose tested, the mean number of leaks was significantly lower in animals treated with MPFF compared with those treated with the same dose of the nonmicronized form (P<0.001).
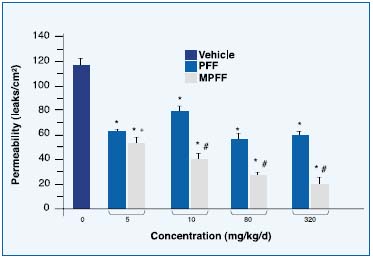
Figure 3. Mean maximum number
of venular leakage sites per cm2 during
reperfusion after 30 min of total
ischemia in the cheek pouch of hamsters
treated orally for 10 days with
micronized purified flavonoid fraction
(MPFF) or nonmicronized PFF
(n = 6 animals per group).
*P<0.0001 compared with vehicletreated
group and # P<0.0001,
+ P<0.001 compared with
the same dose of nonmicronized PFF.
DISCUSSION
As experimental models of venous disease are rare, the activity of MPFF was investigated using a pharmacological model with microvascular alterations similar to what is observed in chronic venous insufficiency. Several studies have been carried out in models of ischemia/ reperfusion,9,10,11,19 inflammation evoked by edemogenic substances,13 oxidant challenge,9,12 venular occlusion,14 and transcapillary fluid shift after postural changes20 to evaluate this disease.
Hamster striated muscle preparation has been studied with pretreatment with MPFF for 8 days in a 4-hour ischemia model where the tissue damage was probably prevented by reducing the number of extravasated leukocytes.21 Two other studies have shown that pretreatment with MPFF reduces the expression of adhesion molecules (ICAM-I) in the rat cremaster exposed to 4 hour ischemia/reperfusion4 and the spontaneous neutrophil activation and expression of CD 62 L (L-Selectin) after 60 minutes of local venular occlusion followed by a 1-hour reperfusion in the rat mesentery without effect on CD 18 or P-Selectin.14
Our results show that MPFF has more effect in reducing microvascular permeability than nonmicronized PFF. Inflammatory reactions could be avoided with less edema formation. Similar results have also been reported in the literature.22,23 In postischemic reperfusion injury, leukocyte activation and adhesion to the endothelium and an increase in macromolecular permeability are a characteristic pattern.9,24,25,26
The pharmacokinetics and metabolism of MPFF could explain the differences between this form and the other one. Johnston and coworkers,27 using 14C in the position 2 of the intermediate ring of diosmin, the major component of MPFF, demonstrated that micronization significantly improved the absorption of a single dose of 10 mg/kg body weight compared with nonmicronized PFF after 168 h of collection (fecal excreted radioactivity – MPFF 21% and nonmicronized PFF 72%; urinary excreted radioactivity – MPFF 77% and nonmicronized PFF 17%).
Another example of effectiveness of the digestive absorption of MPFF is the double-blind, crossover study in which 12 volunteers used 500 mg tablets containing trace amounts of 14C-diosmin in a single oral dose. The reduction of particle size of 14C-diosmin resulted in a marked increase in the proportion of the dose excreted in the urine.28 The increased urinary excretion possibly reflects higher absorption of the micronized formulation giving a pharmacokinetics explanation of its better clinical efficacy.
Our results are in accordance with clinical findings in the treatment of chronic venous insufficiency and hemorrhoidal disease,27,28 where MPFF reduced significantly the symptoms and objective signs of these diseases with equivalent acceptability.27,28
Oral treatment with MPFF, at 20 mg/kg body weight/day for 10 consecutive days significantly decreased the macromolecular permeability increase induced by ischemia/reperfusion (103.6 ± 15.4 versus 42.6 ± 9.3 leaks/cm2, P<0.01).9 The effects of MPFF on microvascular macromolecular permeability increase were confirmed in different models and species.9-13 Leukocyte adhesion to endothelial cells of postcapillary venules, which plays a major role in the pathogenesis of venous disease,29 decreased in various experimental models (ischemia/reperfusion, venular occlusion/reperfusion, oxidant challenge), as demonstrated by intravital microscopy or histomorphological analysis.9,10,12,13,19,22
Some observations suggest leukocyte adhesion may contribute to damage of the microcirculation and, ultimately to the formation of leg ulcers in patients suffering from chronic venous insufficiency.20
In conclusion, micronization significantly enhances the protective effects of oral administration of the purified flavonoid fraction on macromolecular permeability increase induced by ischemia/reperfusion in the hamster cheek pouch preparation. Better absorption of the compound can explain its superior clinical efficacy shown in previous studies.

REFERENCES
2. Struckman JR. Clinical efficacy of micronized MPFF at a dose of 500 mg: an overview. J Vasc Res. 1999;36(suppl 1):37-41.
3. Pearson JD. Pathophysiological mechanisms involving leukocytes in chronic venous insufficiency. Prog Appl Microcirc. 1999;23:82-90.
4. Korthuis RJ, Gute DC. Pathophysiologic implications of leukocyte activation in chronic venous insufficiency. Medicographia. 2000;22:123-132.
5. Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47-92.
6. Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000;7:S29-S34.
7. Kloner CA, Ganote CE, Jennings F. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496-1508.
8. Menger MD, Steiner DR, Meßmer K. Microvascular ischemia-reperfusion in striated muscle: significance of “reflow paradox”. Am J Physiol. 1992;263:H1901- H1906.
9. Bouskela E, Donyo KA. Effects of oral administration of purified micronized flavonoid fraction on increased microvascular permeability induced by various agents and on ischemia/reperfusion in the hamster cheek pouch. Angiology. 1997;48:391-399.
10. Korthuis RJ, Gute DC. Postischemic leukocyte/endothelial cell interactions and microvascular barrier dysfunction in skeletal muscle: cellular mechanisms and effect of MPFF at a dose of 500 mg. Int J Microcirc Clin Exp. 1997;17(suppl 1):11-17.
11. Nolte D, Pickelmann S, Mollman M, Schultze E, Kubler W, Leiderer R, Meßmer K. Effects of the phlebotropic drug MPFF at a dose of 500 mg on postischemic microvascular disturbances in striated skin muscle: an intravital microscopic study in the hamster. J Lab Clin Med. 1999;139:526-535.
12. Bouskela E, Cyrino FZGA, Lerond L. Leukocyte adhesion after oxidant challenge in the hamster cheek pouch microcirculation. J Vasc Res. 1999;36 (suppl 1):11-14.
13. Bouskela E, Donyo KA, Verbeuren TJ. Effects of MPFF at a dose of 500 mg on increased microvascular permeability in normal hamsters. Int J Microcirc Clin Exp. 1995;15(suppl 1):22-26.
14. Takase S, Delano FA, Lerond L, Bergan JJ, Schmid-Schönbein GW. Inflammation in chronic venous insufficiency: is the problem insurmountable? J Vasc Res. 1999;36(suppl 1):3-10.
15. Duling BR. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973;5:423-429.
16. Svensjö E, Arfors KE, Arturson G, Rutili G. The hamster cheek pouch preparation as a model for studies of macromolecular permeability of the microvasculature. Uppsala J Med Sci. 1978;83:71-79.
17. Bouskela E, Grampp W. Spontaneous vasomotion in the hamster cheek pouch arterioles in varying experimental conditions. Am J Physiol. 1992;262: H478-H485.
18. Persson NH, Erlansson M, Svensjö E, Takolander R, Bergqvist D. The hamster cheek pouch – an experimental model to study postischemic macromolecular permeability. Int J Microcirc Clin Exp. 1985;4:257-263.
19. Friesenecker B, Tsai A, Allegra C, Intaglietta M. Oral administration of purified micronized flavonoid fraction suppresses leukocyte adhesion in ischemia/reperfusion injury: in vivo observations in the hamster skinfold. Int J Microcirc Clin Exp. 1994;14:50-55.
20. Coleridge-Smith PD. The microcirculation in venous hypertension. Cardiovasc Res. 1996;72:789-795.
21. Pickelmann S, Nolte D, Leiderer R, Mollmann M, Schultze E, Meßmer K. Effects of the phlebotropic drug MPFF at a dose of 500 mg on postischemic reperfusion injury in striated skin muscle: a histomorphologic study in the hamster. J Lab Clin Med. 1999;134:536-545.
22. Bertuglia S, Colantuoni A, Intaglietta M. Effect of leukocyte adhesion and microvascular permeability on capillary perfusion during ischemia-reperfusion injury in the hamster cheek pouch. Int J Microcirc Clin Exp. 1993;13:13-26.
23. Erlansson M, Bergqvist D, Marklund SL, Persson NH, Svensjö E. Superoxide dismutase as an inhibitor of postischemic microvascular permeability increase in the hamster. Free Radical Biol Med. 1990; 9:59-65.
24. Hultström D, Svensjö E. Intravital and electron microscopic study of bradykinininduced vascular permeability changes using FITC-dextran as a tracer. J Pathol. 1979;129:125 (abstract).
25. Persson CGA, Svensjö E. Vascular responses and their suppression: Drugs interfering with venular permeability. In: Bonta IL, Bray MA, Parnham MJ, eds. Handbook of Inflammation. The Pharmacology of Inflammation. Vol 5. Amsterdam: Elsevier;1985:61-82.
26. He P, Curry FE. Depolarization modulates endothelial cell calcium influx and microvessel permeability. Am J Physiol. 1991;261:H1246-H1254.
27. Cospite M. Dominici A. Advantages de la micronisation de MPFF at a dose of 500 mg par rapport à une diosmine simple dans le traitement de l’insuffisance veineuse. Etude en double aveugle. Phlébologie. 1998;51:243-247.
28. Cospite M, Milio G. Overview of pharmacological treatment of acute hemorrhoids. Phlebolymphology. 2001;31:10-15.
29. Verbeuren TJ, Vallez MO, Rupin A, Wierzbicki M, Cayatte A, Cohen RA, Bouskela E. New synthetic diosmetin derivatives: effects on different experimental models of the microcirculation. Prog Appl Microcirc. 1999;23:104-113.
