Outcomes of different approaches for the treatment of large-diameter incompetent great saphenous veins
Christos S. Karathanos,
MD, PhD
Department of Vascular Surgery,
University Hospital of Larissa,
Faculty of Medicine, School of
Health Sciences, University of
Thessaly, Larissa, Greece
Athanasios D.
Giannoukas,, MD, PhD
Department of Vascular Surgery,
University Hospital of Larissa,
Faculty of Medicine, School of
Health Sciences, University of
Thessaly, Larissa, Greece
ABSTRACT
Chronic venous disease is a common disorder reported to affect up to 60% of the general population. Treatment options include conservative treatment, conventional surgery, and endovenous techniques. Great saphenous vein (GSV) diameter remains a controversial issue when considering optimal treatment, as a limited number of studies included patients with large-diameter GSV. This review focuses on the role of GSV diameter in the outcomes of different approaches for the treatment of incompetent GSV. Endovenous thermal ablation techniques are considered the first-choice treatment, with lower recurrence and complication rates in large-diameter GSV than observed with conventional surgery and nonthermal ablation techniques. Higher laser wavelengths are more effective than lower laser wavelengths in large GSV. Nonthermal ablation techniques seem not to be appropriate treatment for GSV diameters larger than 6 mm.
Introduction
Chronic venous disease (CVD) is a common disorder reported to affect up to 60% of the general population.1 The annual incidence of patients with varicose veins (VVs) ranged from 0.2% to 2.3%, whereas one-third of patients with uncomplicated VVs will develop skin changes and venous ulcers in the next 6 years.2
The symptoms attributed to CVD vary to different degrees of severity, from asymptomatic forms to leg pain, burning sensation, itching, heaviness, nocturnal cramps, skin changes, and ulceration of the limbs, affecting patients’ quality of life (QOL). Symptoms usually increase with age and are more commonly reported in females.2
In patients with VVs, management strategies depend on clinical presentation (symptoms and signs), duplex ultrasound (DUS) findings, complications such as superficial vein thrombosis or hemorrhage, QOL impairment and a patient’s preference.
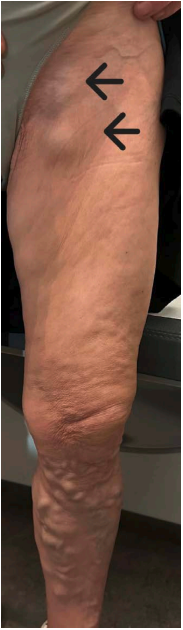
Figure 1. Patient with chronic venous disease of the left limb. Notice the visible large-diameter great saphenous vein.
Photo provided courtesy of the Department of Vascular Surgery, University Hospital of Larissa, Larissa, Greece.
Treatment options include conservative treatment (compression stockings and venoactive drugs), conventional surgery with ligation of the saphenofemoral junction and stripping of the incompetent saphenous vein, and endovenous techniques. Numerous studies have shown the beneficial effect of intervention on venous symptoms, not only in CVD patients presenting with skin changes and venous ulcers (CEAP [clinical etiological-anatomical pathophysiological] clinical class C4 to C6), but also in those with uncomplicated VVs.3,4 Additionally, other studies have shown the cost-effectiveness of interventional treatment versus conservative treatment in these patients.5,6 According to the current guidelines from the European Society for Vascular Surgery, patients with superficial venous incompetence presenting with symptomatic VVs (CEAP clinical class C2S), interventional treatment is recommended (level of evidence A, class I).7
Superficial vein incompetence is mainly attributed to the great saphenous vein (GSV) and its branches. GSV size plays an important role in clinical disease severity and also in postoperative outcomes.8 The concept of large-diameter veins does not have a common definition as there is variation among different studies with regard to reported diameters for the large GSV trunk and in the site where GSV measurement is taken. Reported diameters for the large GSV trunk vary between 8 and 15 mm.9,10 Some studies measure the GSV trunk 3 cm below the saphenofemoral junction (SFJ),11,12 others at the level of the thigh,13 whereas others do not specify the site of measurement at all.14,15 According to the recommendations from the International Union of Phlebology (UIP) consensus document, maximum GSV diameter should be measured on DUS in the standing position, at the level of the thigh, in a tubular part of the trunk, excluding focal dilatation or aneurysms (Figure 1 and Figure 2).16
The diameter of GSV remains a controversial issue when choosing the optimal treatment as there is a limited number of studies that included patients with large-diameter GSV and reported the effectiveness of treatment approaches.
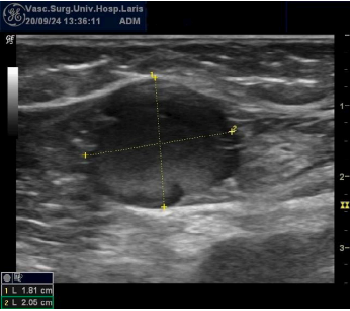
Figure 2. Large-diameter great saphenous vein on duplex ultrasound.
Photo provided courtesy of the Department of Vascular Surgery, University Hospital of Larissa, Larissa, Greece.
Many studies have reported that open surgery should be recommended for large-diameter GSVs as endovenous techniques are associated with higher recurrence and complication rates,17,18 whereas others reported that large GSVs may be treated effectively with endovenous treatment.11,13,19 This review focuses on the role of GSV diameter in the outcomes of different approaches for the treatment of incompetent GSV.
High ligation and stripping
High ligation of the SFJ and stripping (HLS) of the incompetent saphenous vein has been the standard treatment of superficial vein incompetence for many years. Over the past decade, conventional surgery has been substantially replaced by endovenous techniques, although HLS should be considered if endovenous thermal ablation (EVTA) options are not available (level of evidence A, class IIa).7 Two randomized controlled trials (RCT) with long-term outcomes reported a recurrence rate of 4% and 11% after HLS at 5 and 11 years of follow-up, respectively.20,21 A meta-analysis by Hamann et al, found that long-term (5 years) recurrence rates were significantly lower after HLS than after endovenous laser ablation (EVLA) (12%, 95% CI 7%-20% vs 22%, 95% CI 14%- 32%; P=0.038).22 Others reported that long-term results of HLS do not differ from those of EVTA with respect to recurrence.23,24 Nevertheless, HLS is associated with more frequent postoperative complications, such as hematoma, wound infection, paresthesia, and longer hospitalization and recovery than with EVLA.25
Many practitioners consider HLS to be a superior treatment option for large-diameter GSV.17,18 In a retrospective study by Kubat et al comparing 5 different approaches in patients with GSV diameter ≥10 mm, HLS-treated patients had lower recurrence rates compared with 980-nm EVLA, 1470-nm EVLA, radiofrequency ablation (RFA), and cyanoacrylate adhesive closure (CAC).14 Nevertheless, the study concluded that recurrence rates were not statistically significantly different at 6 months and 1 year among HLS, 1470-nm EVLA, and RFA.14 Another multicenter retrospective study including patients with GSV ≥14 mm found that HLS was associated with more adverse events, such as postoperative pain, hemorrhage, and incidence of paresthesia, although recurrence rates were similar to those with RFA.12
Thermal ablation techniques
Since the first EVLA procedure performed in 1999,26 endovenous techniques have become very popular as a minimally invasive alternative procedure to traditional surgery. The two most commonly used EVTA techniques are EVLA and RFA. The recently published European Society for Vascular Surgery guidelines recommend EVTA in preference to surgery and to foam sclerotherapy for the treatment of incompetent GSV (level of evidence A, class I).7 Endovenous steam ablation and endovenous microwave ablation are two alternative EVTA techniques, although there is limited data about these in the literature. The technique is similar for all EVTA methods. The procedure is performed under DUS guidance and requires the use of buffered solutions for tumescent anesthesia. Percutaneously, a laser fiber or RFA catheter is inserted and advanced distal to the SFJ or saphenopopliteal junction. While withdrawing the catheter or fiber, thermal energy is emitted into the vein wall causing endothelial damage and vein occlusion.
Efficacy and safety of EVTA techniques heavily depend on multiple parameters, such as anatomical characteristics, technical device parameters, and proper technique. Initially, low laser wavelengths (hemoglobin targeting) with bare-tip fibers have been replaced by higher laser wavelengths (water targeting) with different configuration fiber tips (radial ring, jacketed tip, tulip tip). In the water-targeting lasers (>1320 nm), the absorption of the energy by the venous wall is higher; thus, by delivering less energy, increased efficiency and reduced complications are achieved. Many studies comparing 980-nm with 1470-nm fibers reported that the higher wavelength was associated with comparable occlusion rates, less postoperative pain, ecchymosis, paresthesia, and induration.27-29 Similarly, the second-generation RFA catheters are more efficient and safer than first-generation catheters, as the thermocouple is enclosed in a lubricated sheath that ensures obliteration and prevents target-vein carbonization and thrombosis.13,30
Previous EVTA studies reported that larger GSVs had lower occlusion rates and higher complication rates, including endothermal heat-induced thrombosis (EHIT), although the latter conclusion was mainly based on patients treated with hemoglobin-targeting laser fibers and first-generation RFA catheters.17,18,31 A prospective comparative study, including GSV >15 mm, displayed excellent occlusion (95%) and healing ulcer rates (88%) in patients treated with the 1560-nm EVLA wavelength.32 Another retrospective study, including GSV >10 mm, found similar recurrence rates among 1470-nm EVLA, RFA, and HLS (5.5%, 5.7%, and 3.3%, respectively) at 1-year follow-up.14 In addition, the recurrence rates of EVLA at the 980-nm wavelength and of CAC-treated patients were higher than in other groups (14.6% and 15.2%).14 A prospective comparative study, comparing 1470-nm EVLA and RFA in patients with GSV >10 mm, reported comparable occlusion rates, although there were lower complication rates in the 1470-nm EVLA group, such as postoperative pain and ecchymosis.15 Another unpublished study, presented at the UIP 2023 World Congress and American Vein & Lymphatic Society (AVLS) 2023 Annual Congress, reported that 1470-nm EVLA and RFA in patients with GSV >12 mm had comparable results in terms of occlusion rates, complications, venous clinical severity score (VCSS) and Chronic Venous Insufficiency Quality-of-Life Questionnaire (CIVIQ) scores.33 Two more studies, investigating the efficacy of RFA in large diameter GSV >12 mm, found high occlusion rates (96% and 100%) and low complication rates (8% and 13.6%) at 1-year follow-up.11,13 A recent systematic review and meta-analysis by Bontinis et al reported excellent occlusion (95.9%) and technical success rates (99.9%) for the EVTA of GSV >12 mm.34 Furthermore, the study found no association between occlusion rates, the type of device used, and the length of follow-up.34
Although there is controversy around EVTA techniques and large-diameter GSV, newer-generation devices, variable application of energy and tumescent anesthesia, external compression and multi-pass technique during ablation, and also closer surveillance for early detection of complications have increased the efficacy and safety of EVTA.10,11,19,33 Current guidelines recommend that in patients with an incompetent GSV >12 mm, EVTA should be considered (level of evidence IIa, class C).7
Nonthermal ablation techniques
Nonthermal endovenous techniques are ultrasound-guided foam sclerotherapy (UGFS), mechanochemical ablation (MOCA), and catheter-directed injection of cyanoacrylate glue, known as CAC. There are many similarities among these treatments, such as saphenous vein cannulation, endovenous substance infusion, and no need for tumescent anesthesia.
During UGFS, a sclerosing agent, most commonly polidocanol or sodium tetradecyl sulphate in various concentrations, is injected into the target vein to cause fibrosis of the vein. Many studies with long-term follow-up have shown that recurrence rates are higher in patients treated with UGFS than with EVTA and HLS.35,36 Nevertheless, the advantages of UGFS are that it can be easily applied for tortuous veins where there are difficulties in advancing the ablation device and it’s suitable for recurrent VVs. An alternative to the classical UGFS is catheter-directed foam sclerotherapy (CDFS) with or without tumescent anesthesia in order to reduce the vein caliber. A systematic review and meta-analysis showed a higher occlusion rate of 82.4% after CDFS and 62.9% after UGFS at 3-year follow-up.37 Regarding GSV diameter, Shadid et al, reported higher recurrence rates after UGFS in patients with mid-thigh GSV diameter >6 mm (62.6%) versus smaller ones (42%) at 2-year follow-up.38 Another study also reported worse success rates for veins >6 mm (hazard ratio [HR] 2.22; 95% CI 1.40-3.50) compared with veins <5 mm.39 Venermo et al also found an association between larger-diameter and GSV patency.40 The occlusion rate after UGFS was less than 40% in mid-thigh GSVs ≥9 mm compared with 75% in GSVs <6 mm.40 Therefore, UGFS should be preferably used for veins smaller than 6 mm in diameter.38-40
The MOCA technique uses a dual-injury mechanism that combines mechanical disruption of the intima with chemical endovenous ablation. Damage of the endothelium is achieved through a rotating wire or sharp hook at the tip of the catheter while chemical ablation is performed by injecting a foam sclerosant. A systematic review and meta-analysis have shown that the pooled anatomic success after MOCA was 94.1% at 1-year follow-up.41 One RCT reporting outcomes at 3 years found a significantly lower occlusion rate after MOCA than with EVTA (80% vs 100%).42 The study also found a strong association between recanalization and GSV diameter. The occlusion rates for a preoperative GSV diameter of 6 mm, 7 mm, and 8 mm were 100%, 87.5%, and 75%, respectively.42
Upon CAC, intravenous injection of cyanoacrylate rapidly solidifies via a polymerization reaction and produces an inflammatory reaction of the vein wall. Currently, 3 types of CAC devices are commercially available, and the main differences relate to the cyanoacrylate formulation and application techniques. Several studies have shown that CAC is safe and effective to ablate the incompetent GSV, with cumulative occlusion rates comparable to those for EVTA and better compared with other nonthermal ablation techniques (up to 93.6% at 5 years).42-44 For patients with superficial venous incompetence of a saphenous trunk requiring treatment, CAC should be considered when a nonthermal nontumescent technique is preferred (level of evidence IIa, class A).7 Chan et al, found that a mean GSV diameter ≥8mm was a significant predicting factor for recanalization (HR, 6.92; 95% CI, 1.34–35.67; P=0.021).45 A saphenous vein diameter of >8 mm has also been reported as a risk factor for hypersensitivity reaction after CAC.46 Another study reported that in patients with GSV >10 mm, 1-year recurrence rates with CAC were higher than with 1470-nm EVLA and RFA (15.2%, 5.5%, and 5.7%, respectively).14 A network meta-analysis on the efficacy and safety of thermal and nonthermal endovenous ablation treatments found a trend for a considerably decreased efficacy with both CAC and MOCA than with RFA and EVLA for larger GSV diameters.47
Conclusions
Interventional treatment remains the optimal therapy for patients with superficial venous incompetence presenting with symptomatic VVs. EVTA techniques are considered the first-choice treatment regardless of GSV diameter. Higher laser wavelengths are more effective than lower laser wavelengths in large GSV. Nonthermal ablation techniques seem to be inappropriate treatment for GSV diameters larger than 6 mm.

CORRESPONDING AUTHOR
Christos S. Karathanos, MD, MSc, PhD
Department of Vascular Surgery,
University Hospital of Larissa, Mezourlo,
41 110 Larissa, Greece
EMAIL: christoskarathanos@yahoo.gr

CORRESPONDING AUTHOR
Athanasios D. Giannoukas, MD, PhD
Department of Vascular Surgery,
University Hospital of Larissa, Faculty
of Medicine, School of Health Sciences,
University of Thessaly, Larissa, Greece
References
1. Robertson L, Evans C, Fowkes FG. Epidemiology of chronic venous disease. Phlebology. 2008;23(3): 103‐111.
2. Lee AJ, Robertson LA, Boghossian SM, et al. Progression of varicose veins and chronic venous insufficiency in the general population in the Edinburgh Vein Study. J Vasc Surg Venous Lymphat Disord. 2015;3: 18-26.
3. Carradice D, Wallace T, Gohil R, Chetter I. A comparison of the effectiveness of treating those with and without the complications of superficial venous insufficiency. Ann Surg. 2014;260: 396- 401.
4. Michaels JA, Brazier JE, Campbell WB, MacIntyre JB, Palfreyman SJ, Ratcliffe J. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg. 2006;93: 175-181.
5. Ratcliffe J, Brazier JE, Campbell WB, Palfreyman S, MacIntyre JB, Michaels JA. Cost-effectiveness analysis of surgery versus conservative treatment for uncomplicated varicose veins in a randomized clinical trial. Br J Surg. 2006;93: 182-186.
6. Marsden G, Perry M, Bradbury A, et al. A cost-effectiveness analysis of surgery, endothermal ablation, ultrasound-guided foam sclerotherapy and compression stockings for symptomatic varicose veins. Eur J Vasc Endovasc Surg. 2015;50: 794- 801.
7. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63: 184- 267.
8. Lane TR, Varatharajan L, Fiorentino F, et al. Truncal varicose vein diameter and patient reported outcome measures. Br J Surg. 2017;104: 1648-1655.
9. Hamel-Desnos CM, De Maeseneer M, Josnin M, Gillet JL, Allaert FA; DIAGRAVES Study Group. Great saphenous vein diameters in phlebological practice in France: a report of the DIAGRAVES Study by the French Society of Phlebology. Eur J Vasc Endovasc Surg. 2019;58: 96-103.
10. Dabbs EB, Mainsiouw LE, Holdstock JM, Price BA, Whiteley MS. A description of the ‘smile sign’ and multi-pass technique for endovenous laser ablation of large diameter great saphenous veins. Phlebology. 2018;33(8): 534-539.
11. Woo HY, Kim SM, Kim D, Chung JK, Jung IM. Outcome of ClosureFAST radiofrequency ablation for large-diameter incompetent great saphenous vein. Ann Surg Treat Res. 2019;96: 313-318.
12. Shaidakov EV, Grigoryan AG, Ilyukhin EA, Bulatov VL, Rosukhovskiy DA. Radiofrequency ablation or stripping of large-diameter incompetent great saphenous varicose veins with C2 or C3 disease. J Vasc Surg Venous Lymphat Disord. 2016;4: 45-50.
13. Fernandez MC, Lopez IM, Hernandez Mateo MM, Marques de Marino P, Artero IC, Serrano Hernando FJS. Prospective study of safety and effectiveness in the use of radiofrequency ablation for incompetent great saphenous vein >12 mm. J Vasc Surg Venous Lymphat Disord. 2017;5: 810-816.
14. Kubat E, Ünal CS, Geldi O, Çetin E, Keskin A. What is the optimal treatment technique for great saphenous vein diameter of ≥10 mm? Comparison of five different approaches. Acta Chirurgica Belgica. 2021;121(2): 94-101.
15. Mese B, Bozoglan O, Eroglu E, et al. A comparison of 1470 nm endovenous laser ablation and radiofrequency ablation in the treatment of great saphenous veins 10 mm or more in size. Ann Vasc Surg. 2015;29(7): 1368-1372.
16. Maeseneer M, Pichot O, Cavezzi A, et al. Duplex ultrasound investigation of the veins of the lower limbs after treatment of varicose veins UIP consensus document. Eur J Vasc Endovasc Surg. 2011;42: 89- 102.
17. van den Bos R, Neumann M, de Roos K, et al. Endovenous laser ablation-induced complications: review of the literature and new cases. Dermatol Surg. 2009;35: 1206- 1214.
18. Corcos L, Dini S, Peruzzi C, et al. Duplex ultrasound changes in the great saphenous vein after endosaphenous laser occlusion with 808-nm wavelength. J Vasc Surg. 2008;48: 1262-1271.
19. Atasoy M. Efficacy and safety of endovenous laser ablation in very large and tortuous great saphenous veins. J Vasc Interv Radiol. 2015;26: 1347-1352.
20. Dwerryhouse S, Davies B, Harradine K, et al. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg. 1999;29(4): 589-592.
21. Winterborn RJ, Foy C, Earnshaw JJ. Causes of varicose vein recurrence: late results of a randomized controlled trial of stripping the long saphenous vein. J Vasc Surg. 2004;40: 634-639.
22. Hamann SAS, Giang J, De Maeseneer MGR, Nijsten TEC, van denBos RR. Editor’s Choice – Five year results of great saphenous vein treatment: a meta-analysis. Eur J Vasc Endovasc Surg. 2017;54: 760-770.
23. Whing J, Nandhra S, Nesbitt C, Stansby G. Interventions for great saphenous vein incompetence. Cochrane Database Syst Rev. 2021;8: CD005624.
24. Kheirelseid EAH, Crowe G, Sehgal R, et al. Systematic review and meta-analysis of randomized controlled trials evaluating long term outcomes of endovenous management of lower extremity varicose veins. J Vasc Surg Venous Lymphat Disord. 2018;6: 256-270.
25. Pan Y, Zhao J, Mei J, Shao M, Zhang J. Comparison of endovenous laser ablation and high ligation and stripping for varicose vein treatment: a meta-analysis. Phlebology. 2014;29: 109-119.
26. Boné SC. Tratamiento Endoluminal de las varices con laser de diodo. Estudio preliminar. Patologia Vascular. 1999;5: 32- 39.
27. Doganci S, Demirkilic U. Comparison of 980 nm laser and bare tip fibre with 1470 nm laser and radial fibre in the treatment of great saphenous vein varicosities: a prospective randomized clinical trial. Eur J Vasc Endovasc Surg. 2010;40: 254-259.
28. Hirokawa M, Ogawa T, Sugawara H, Shokoku S, Sato S. Comparison of 1470 nm laser and radial 2ring fiber with 980 nm laser and bare-tip fiber in endovenous laser ablation of saphenous varicose veins: a multicenter, prospective, randomized, non-blind study. Ann Vasc Dis. 2015;8: 282-289.
29. Arslan U, Calik E, Tort M, et al. More successful results with less energy in endovenous laser ablation treatment: long-term comparison of bare-tip fiber 980 nm laser and radial-tip fiber 1470 nm laser application. Ann Vasc Surg. 2017;45: 166-172.
30. Zuniga JM, Hingorani A, Ascher E, et al. Short term outcome analysis of radiofrequency ablation using ClosurePlus vs ClosureFast catheters in the treatment of incompetent great saphenous vein. J Vasc Surg. 2012;55: 1048-1051.
31. Gloviczki P, Comerota AJ, Michael C, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(Suppl): 2S-48S.
32. Starodubtsev V, Lukyanenko M, Karpenko A, Ignatenko P. Endovenous laser ablation in patients with severe primary chronic venous insufficiency. Int Angiol. 2017;36: 368-374.
33. Karathanos C, Spanos K, Batzalexis K, et al. Endovenous thermal ablation techniques for the treatment of large diameter incompetent great saphenous veins. Paper presented at: Union International Phlebology 2023 World Congress and American Vein and Lymphatic Society; September 17-21, 2023; Miami, Florida, USA.
34. Bontinis V, Bontinis A, Koutsoumpelis A, et al. Endovenous thermal ablation in the treatment of large great saphenous veins of diameters > 12 mm: a systematic review meta-analysis and meta-regression. Vasc Med. 2023;28(5):449- 457.
35. Brittenden J, Cooper D, Dimitrova M, et al. Five-year outcomes of a randomized trial of treatments for varicose veins. N Engl J Med. 2019;381:912-922.
36. van der Velden SK, Biemans AA, De Maeseneer MG, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. Br J Surg. 2015;102: 1184-1194.
37. Lim SY, Tan JX, D’Cruz RT, Syn N, Chong TT, Tang TY. Catheter-directed foam sclerotherapy, an alternative to ultrasound-guided foam sclerotherapy for varicose vein treatment: a systematic review and meta-analysis. Phlebology. 2020;35:369-383.
38. Shadid N, Nelemans P, Lawson J, Sommer A. Predictors of recurrence of great saphenous vein reflux following treatment with ultrasound-guided foam sclerotherapy. Phlebology. 2015;30:194- 199.
39. Myers KA, Jolley D, Clough A, Kirwan J. Outcome of ultrasound guided sclerotherapy for varicose veins: medium-term results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33:116-121.
40. Venermo M, Saarinen J, Eskelinen E, et al. Randomized clinical trial comparing surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy for the treatment of great saphenous varicose veins. Br J Surg. 2016;103:1438-1444.
41. Vos CG, Unlu C, Bosma J, van Vlijmen CJ, de Nie AJ, Schreve MA. A systematic review and meta-analysis of two novel techniques of non thermal endovenous ablation of the great saphenous vein. J Vasc Surg Venous Lymphat Disord. 2017;5:880-896.
42. Vähäaho S, Halmesmäki K, Mahmoud O, Albäck A, Noronen K, Venermo M. Three-year results of a randomized controlled trial comparing mechanochemical and thermal ablation in the treatment of insufficient great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2021;9:652- 659.
43. Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020;8:978-989.
44. Garcia-Carpintero E, Carmona M, Chalco Orrego JP, Gonzalez-Enriquez J, Imaz Iglesia I. Systematic review and meta analysis of endovenous cyanoacrylate adhesive ablation for incompetent saphenous veins. J Vasc Surg Venous Lymphat Disord. 2020;8:287-296.
45. Chan YC, Law Y, Cheung GC, Ting AC, Cheng SW. Cyanoacrylate glue used to treat great saphenous reflux: measures of outcome. Phlebology. 2017;32:99-106.
46. Sermsathanasawadi N, Hanaroonsomboon P, Pruekprasert K, et al. Hypersensitivity reaction after cyanoacrylate closure of incompetent saphenous veins in patients with chronic venous disease: a retrospective study. J Vasc Surg Venous Lymphat Disord. 2021;9:910-915.
47. Bontinis V, Bontinis A, Koutsoumpelis A, et al. A network meta-analysis on the efficacy and safety of thermal and nonthermal endovenous ablation treatments. J Vasc Surg Venous Lymphat Disord. 2023;11(4):854-865.
