Optimal management of combined iliac vein stenosis and ovarian vein reflux in patients with pelvic venous disease (PeVD): which patients should be treated for both and what comes first?
Karem Harth, MD, MHS, RPVI
Director, Center for Comprehensive
Venous Care University Hospital
Harrington Heart & Vascular
Institute, Associate Professor
of Surgery, Division of Vascular
Surgery and Endovascular Therapy,
Case Western Reserve University
School of Medicine, Cleveland, Ohio,
United States
Gloria Salazar, MD
Associate Clinical Professor
Radiology, Division of Vascular
Interventional Radiology,
University of North Carolina at
Chapel Hill, Chapel Hill, North
Carolina, United States
Introduction
The increased recognition of iliac vein stenosis in the causative effect of venous origin–chronic pelvic pain has led to an ongoing debate of the evaluation, diagnosis, and treatment of patients with pelvic venous disease (PeVD) who present with both iliac venous stenosis and ovarian vein reflux. While ovarian reflux has been thought to play a primary role in the pathophysiology of this disease process, increased evidence suggests that iliac vein stenosis can also lead to chronic pelvic pain and other associated conditions present in these patients, such as chronic venous disease and postural orthostatic tachycardia. Moreover, patient evaluation is confronted by associated nonspecific pelvic symptoms and various anatomical presentations, which are becoming more diagnosable. This is leading to an increase in incidence of patients presenting with combined patterns, which poses a challenge in choosing a treatment algorithm. This article will describe insights to accurately diagnose the disease with its different clinical and anatomical presentations, focusing on the management of combined iliac vein stenosis and ovarian vein reflux in patients with nonthrombotic disease.
Introduction
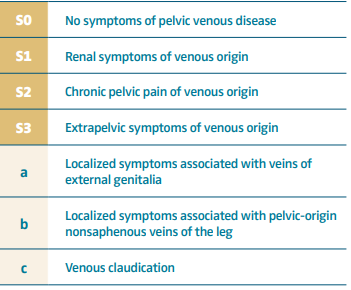
Pelvic venous disease (PeVD) is defined as the spectrum of signs and symptoms secondary to abnormal venous flow in the pelvic area. However, it remains poorly understood and frequently misdiagnosed, partly due to nonstandard anatomical terminology and limited diagnostic criteria for the venous conditions it encompasses.1 This issue is further complicated by the presence of “mixed” anatomical pathways implicated in the development of venous origin–chronic pelvic pain (VO-CPP), highlighting the varied presentations of disease state in different patients. Recently, the Symptoms Varices-Pathophysiology (SVP) classification of pelvic venous disorders was created to improve patient categorization for further understanding of causative factors leading to VO CPP.2 Moreover, there is increased evidence of comorbidities associated with this entity (interstitial cystitis and postural orthostatic tachycardia), that may further contribute to the patients’ clinical presentation.3
Given all these anatomical and clinical contributors to VO-CPP, the interventional treatment of PeVD needs to be optimized for each patient and may involve several treatments needed to achieve clinical success. This may include the following procedures: ovarian vein embolization (OVE), internal iliac vein embolization (IVE), and venous stent placement. Whereas the pathophysiology contributing to the patient’s symptoms will determine the best endovascular treatment, a major issue relates to the different clinical presentations in women of various ages4 and the need for venous stent placement occurring more commonly in older patients.5,6 In this article, we will describe how to evaluate these patients and discuss treatment strategies in nonthrombotic patients with both ovarian vein reflux (OVR) and iliac vein stenosis (IVS).
Anatomy and pathophysiology of chronic pelvic pain
in compression syndromes
in compression syndromes
The SVP classification defines PeVD as the spectrum of symptoms and signs arising from the veins of the pelvis (gonadal veins, internal iliac veins and their tributaries, venous plexuses of the pelvis) and their primary drainage pathways (left renal vein, iliac veins, and pelvic escape points).2 Traditionally, PeVD has been described in patients with pelvic venous reflux (ovarian and internal iliac veins) leading to resultant pelvic varices around the periuterine area.
The uterine plexus is drained by 4 primary veins, the lower portion of the uterus is drained through the bilateral internal iliac veins, and the upper portion of the uterus is drained through the uterine or the ovarian plexus to the ovarian veins (OVs). The left OV drains directly into the left renal vein (Figure 1 A, B), whereas the right OV drains at an acute angle into the inferior vena cava (Figure 2) in most cases. These pelvic veins typically are extensively collateralized with connections with the superficial veins of the genitalia and the lower extremities, which can result in extra-pelvic s y m p t o m s — “ s o called” S3 symptoms in the SVP classification system (Table I).
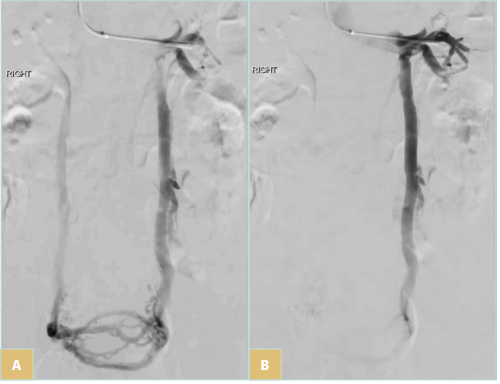
Figure 1. A) Uterine/ ovarian plexus drainage to bilateral gonadal veins. B) Left gonadal vein drainage.
Recently, studies have challenged the causative effect of OVR in PeVD, given that OVR is symptomatic in only up to 59% of patients,7,8 and some evidence shows that iliac vein outflow abnormalities such as stenosis, not OVR, are more common underlying causes of PeVD.5,9,10 One large case series of 1280 patients found that IVS was present in 53% of patients with PeVD, whereas 40% had IVS in combination with OVR.5 In another report of 421 patients, Larkin et al found that 46.7% had iliac vein obstruction, and 40.1% had OV incompetence.9 However, of those who had chronic pelvic pain (CPP), common iliac vein (CIV) obstruction, with and without gonadal vein incompetence (33% and 35%, respectively), was more common than OV incompetence (14%).9 In cases with combined iliac and gonadal venous abnormalities, the OV is acting as a compensatory escape vein rather than the primary pathologic vein.10 This has been supported by a small study demonstrating the resolution or improvement of symptoms in patients with combined IVS and gonadal vein reflux with iliac stenting alone.5 Moreover, IVS has been reported as a cause of intractable pelvic neuropathic pain, in patients with PeVD who had evidence of left OVR and IVS, suggesting a correlation to CPP.11 Lastly, a recent study demonstrated that 69% of female patients with postural orthostatic tachycardia syndrome (POTS) had significant left CIV compression and clinical improvement of dysautonomia symptoms after treatment for their PeVD.12
When should we consider the possibility of iliac vein
stenosis in patients with PeVD?
stenosis in patients with PeVD?
The major clinical challenge in the proper diagnosis of combined patterns is to obtain a proper clinical history and evaluation of presenting symptoms, with patients being categorized in 2 major groups according to the SVP classification system (Table I): S2 and S2,3 patients.2
Consensus documents define the following symptoms: pelvic pain, perineal heaviness, urgency of micturition, and postcoital pain (S2 symptoms), caused by ovarian and/or pelvic vein reflux and/or obstruction, and which may be associated with vulvar, perineal, and/or lower extremity varices (S3 symptoms).13 In addition, symptoms can range from CPP (S2) and renal manifestations (S1 symptoms), if one considers left renal vein compression.14 However, renal symptoms (S1) such as hematuria, dysuria, and urinary frequency in the absence of infection can be present in women with PeVD without left renal vein compression.14 However, if patients report hematuria and or left flank pain, it is important to rule out left renal vein stenosis for proper management of symptoms.
Regardless, the classic description of PeVD includes CPP with dyspareunia and postcoital pain with worsening after prolonged periods of standing (postural pain). It is important to ask patients about the temporal relationship of onset of symptoms and pregnancies, whether the patient has developed vulvar varices or not during that time, and finally, the venous characteristic of the pain: postural CPP (heaviness, cramping) relieved by lying flat, with some patients needing to have a “break” in the middle of the day to relieve the pain. The 3 “P” s (pelvic pain, provoked by gravity, and prolonged postcoital ache) are the most frequent clinical findings in VO-CPP in women, based on the literature.15 Whereas most patients presenting with S2 symptoms have overlapping OVR and IVS, patients with combined venous claudication and edema and or varicose veins in the leg should be evaluated with imaging and ruled out for venous outflow obstruction.
Imaging evaluation and treatment planning
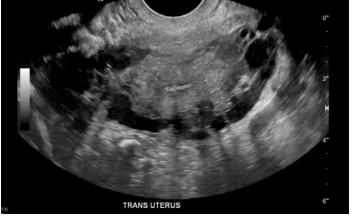
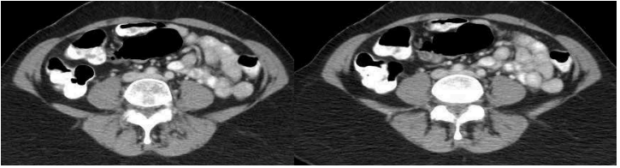
Most patients with CPP will have already undergone limited evaluation with transvaginal ultrasonography (TVUS) (Figures 3 and 4). Most recently, a study demonstrated that the presence of pelvic veins or venous plexus of 8 mm or larger, observed by TVUS, can predict 79% of patients with pelvic congestive syndrome.16 Whereas there is a high association of VO-CPP and evidence of periuterine veins of diameters larger than 8 mm in TVUS, when suspecting iliac stenosis in addition to reflux, the ideal imaging tool for assessment includes either an abdominal ultrasound and/ or cross-sectional imaging, such as computed tomography (CT) venography and magnetic resonance venography (MRV), to specifically diagnose stenosis. The imaging choice will depend on local availability and ultrasonography expertise. There are published ultrasound protocols to evaluate both pelvic venous reflux and stenosis17; however, patient body habitus and presence of overlaying bowel gas can limit the evaluation in about 50% of patients.18 In these instances, CT offers superior spatial resolution over ultrasound, and the use of intravenous contrast can improve characterization of the pelvic vasculature (Figure 5). A drawback is the supine positioning for acquiring the study, which prevents recreation of flow dynamics seen in venous reflux. However, data suggest a significant correlation between left iliac vein cross-sectional area and diameter ratios to reflux start-up time in digital subtraction venography.19 Of note, a 50% or greater iliac stenosis may be present in 25% to 33% of the population. Therefore, anatomic stenosis alone should not be considered a criterion for intervention, and presence of stenosis should be interpreted in the context of patients’ clinical symptoms.20,21
Time-resolved contrast-enhanced MRV does allow for dynamic evaluation of the direction of flow, but MR venography provides a high 42% false-positive rate22; moreover, dehydration status can mimic stenosis, resulting in false positive exams.23 Despite these drawbacks, MRV is favorable in situations where ultrasound imaging is unsuccessful, such as in patients with large body habitus.
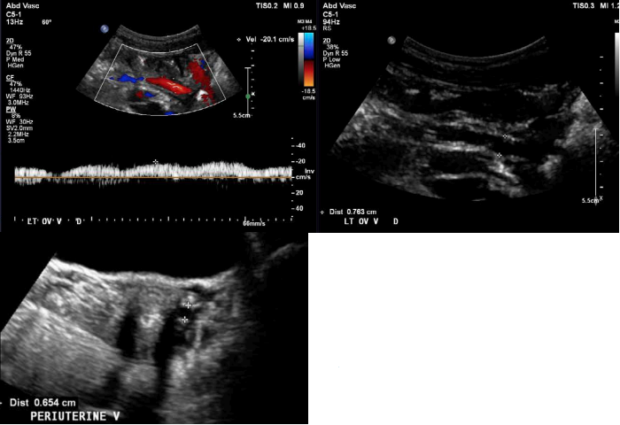
Figure 4. S2, S3a,b patient (according to Symptoms-Varices-Pathophysiology classification) with left ovarian >vein reflux measuring 7.6 mm (top panel). Associated periuterine varices measuring 6.5 mm in diameter (bottom panel).
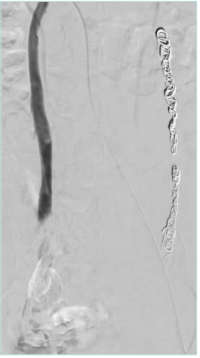
Catheter venography has emerged as the standard of care in diagnosing PeVD, but due to advances in noninvasive imaging and ionizing radiation, it is typically indicated for definitive imaging at the time of treatment. Generally, a catheter venography study involves the placement of a diagnostic catheter into high-frequency points of obstruction where contrast is injected to image for potential areas of compression, scale magnitude of distention, reflux, and pooling (Figure 6). Diagnostic criteria for pelvic varices on catheter venogram include 5 mm or greater diameter of gonadal vein, uterine vein, and utero-ovarian arcade. Free reflux in the gonadal vein with valvular incompetence or filling of contrast material across the midline are also diagnostic criteria (Figure 1B).24 Additionally, described criteria include timing duration of contrast pooling after venography.25 For the diagnosis of IVS with associated reflux, there has been description of a grading system, varying from normal findings (Grade 0) to presence of internal iliac venous reflux (Grade 1), pelvic venous collaterals (Grade 2), and iliolumbar venous collaterals (Grade 3).19
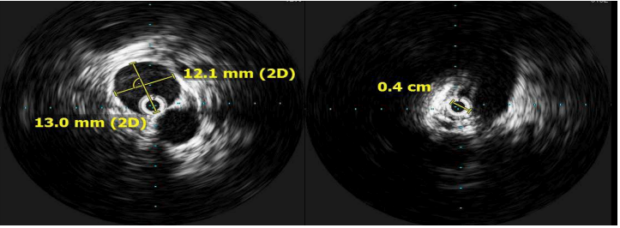
The use of intravascular ultrasound (IVUS), which provides additional information regarding the severity of stenosis (Figure 7), is considered the gold standard for diagnosing CIV compression and planning of iliac vein stenting; however, adequate imaging thresholds correlating with clinical outcomes are lacking.26 In the VIDIO study (Venogram vs IVUS for Diagnosing Iliac vein Obstruction),27 Gagne et al evaluated IVUS stenosis and outcomes, indicating that diameter stenosis was the only significant predictor of future improvement in clinical symptoms in iliac stenosis, with a threshold of >61% by IVUS.27 In addition to being superior to single-plane venography, IVUS is helpful in assessing lesion response to angioplasty, guiding stent placement, and identifying in-stent stenosis.28
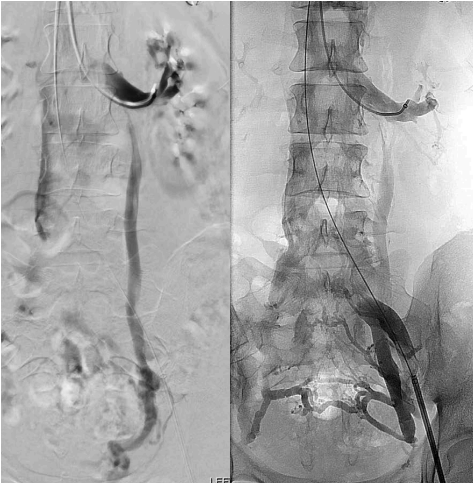
Figure 6. S2 patient (according to
Symptoms-Varices-Pathophysiology
classification) with combined
presentation of ovarian vein reflux
(OVR) and iliac vein stenting (IVS).
Therapeutic considerations
When creating a treatment plan, it is important to note that many patients with PeVD have multiple venous abnormalities contributing to their symptoms, and effective treatment defined by symptomatic improvement or resolution is dependent on the identification of the underlying venous conditions contributing to the patient’s presentation. Careful assessment for presence of the previously described patterns that contribute to this disease, including OV incompetence/reflux, venous stenosis, escape points, and anatomical variant, are critical for effective treatment and determining the treatment plan in each patient. Most patients present with a combination of these pathophysiologic variants, so optimization of treatment strategies is critically important.4
The reality for several patients is that a large proportion have more than one of the above abnormalities contributing to their symptoms. Initial data based on 19 patients suggested that in patients with both common IVS and left OVR, addressing the venous stenosis alone with a stent placement was effective in symptom improvement in most patients.29 However, a more recent study including 12 patients reported symptomatic relief in only 16.6% of patients with PeVD when only stenting was performed and indicated that OVE should be performed 6 months after stenting.30 These findings supported the data from a previous study involving a larger number of patients (n=277) published in 2018, which found that 80% of patients had both OV insufficiency and iliac vein compression. The authors advocated for the treatment of venous stenosis first, followed by OVE if symptoms persisted, but suggested simultaneous treatment in patients with large pelvic reservoirs.10
The order of and timing of optimal treatment is not yet fully elucidated, and there are heterogeneous practice patterns. Below we describe the most current literature on this subject.
Current evidence of literature supporting ovarian
vein embolization first
vein embolization first
Despite extensive publications highlighting the role of OVE in isolated nonobstructive OVR, there is growing evidence demonstrating the role of left iliac vein stenting, challenging whether the primary source of pelvic varices (periuterine varices) and symptoms are secondary to OVR or IVS.9 The role of OVE as a treatment option for PeVD, demonstrates a technical success rate ranging from 96.7% to 100%, and clinical improvement of pain between 50% and 100%,31 with recurrence in up to 25% of patients32 in patients with isolated left OVR. Whereas no large randomized control trials exist comparing percutaneous embolization with medical and or surgical therapies, OVE has emerged as a treatment of choice due to low morbidity, effectiveness, and durability, and is usually the first approach in patients with combined patterns, with stenting being performed at later stages if patients present with persistent VO-CPP.4 Part of the challenge relates to the lack of understanding of the primary physiologic mechanism responsible for VO-CPP in these patients. Moreover, OVR or IVS grading has been based on venographic/imaging criteria and not appropriately defined yet,1 without a quantitative tool to reliably assess hemodynamic flow in these mixed patterns. That said, a few studies retrospectively described the role of OVE first. In a subset of patients with staged OVE and stent (n=94), only 9 patients presented with symptomatic improvement after OVE alone, and most patients required additional venous stenting.10 These results are contrasted by the smaller, above mentioned study reporting symptom relief in only 16.6% with stenting alone and that recommended additional OVE at 6 months for these patients.30
In another study of 43 women with PeVD, authors found the following anatomical distribution of lesions: OVR (61%), internal iliac vein reflux (9%), reflux combination of both (30%), and 42% of patients with anatomical obstruction (inferior vena cava, common iliac or left renal veins). Treatments among these patients included OVE (86%), internal IVE (9%), and venous stent placement (35%), with subsequent improvement in the leading symptom of pelvic pain in up to 93% of patients, whereas 14 (33%) became symptom free.33
Current evidence of literature supporting
stenting first
stenting first
Most of the data published represents a total of 254 women presenting with VO-CPP as a predominant symptom. Associated chronic venous disease (S2,3 patients) was present in about 78% of patients (n=199), of which 27% (n=69) were treated primarily with CIV stent alone.10,29,30
In one of the first series to address iliac vein lesions in PeVD, CIV stent placement was effective in the treatment of CPP even if there was observed untreated left OVR.29 As previously mentioned, a larger study from 201810 in patients with combined patterns suggested that pelvic venous outflow lesions should be treated first, with a subset of patients with large pelvic reservoir needing to have simultaneous treatment with both iliac vein stenting and OVE. However, as mentioned above, a smaller study reported symptom relief in only 16.6% with stenting alone and recommended additional OVE at 6 months.30
Stenting in the setting of PeVD is performed in the same fashion as described for patients with nonthrombotic IVS. The technique has changed with the advent of dedicated venous stents, but in short, the left femoral vein is accessed with a 9F introducer sheath, and an IVUS system is utilized to confirm venous stenosis and estimate the size of stent to be placed based on intraluminal measurements. The assessment begins at the inferior vena cava and continues into both CIVs, external iliac veins, common femoral veins, and femoral veins. In addition, multiplanar venography is performed from the left femoral vein access. Lesions are treated first with balloon angioplasty with an appropriate-length balloon of 12 mm to 14 mm in diameter, and then stenting. Stent sizing is based on the landing venous zone diameters (normal vessel), extending just to the iliac confluence, usually at L4, using IVUS guidance for proper length and diameter, typically measuring 14 mm in diameter with minimum length of 100 mm to avoid stent migration34 and allows for proper positioning within the pelvic vasculature (Figure 8). Stent placement has been associated with back pain in up to 66% of patients 1 week after stent placement, with improvement of symptoms at 1 month.35
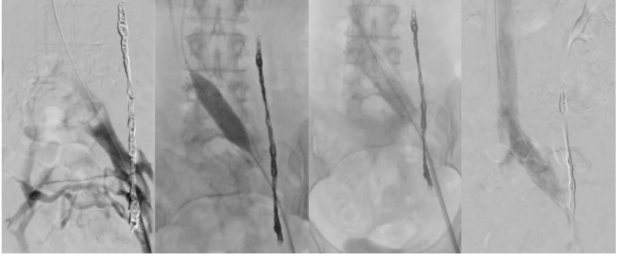
Figure 8. Procedural steps for stent placement. Venography shows Grade 2 reflux and a 14-mm dedicated venous stent placement after left ovarian vein embolization (OVE) in an S2 patient (according to Symptoms-Varices-Pathophysiology classification) with persistent chronic pelvic pain.
Despite most data regarding venous stenting arising from the chronic venous disease literature, data suggesting the effectiveness of venous stenting in patients with VO-CPP as a major manifestation of PeVD is emerging. It is debated whether stenting of the iliac vein alone results in successful outcomes or if other interventions are needed for significant clinical improvement. Therefore, stenting should be done in symptomatic patients whose symptoms are attributable to the stenosis (S2 and/or S2,3 patients), particularly in the younger patient population.36 This is determined through a combination of history, medical work-up, and imaging. Since there is a high prevalence of IVS in asymptomatic patients, it can be a challenge to determine the degree of stenosis that becomes clinically relevant, and treatment would result in symptomatic relief in those with PeVD when there are multiple factors at play.37
Nevertheless, in a small series of 38 women with VO-CPP and chronic venous disease secondary to combined IVS and OVR, 76% of patients achieved complete symptom resolution with iliac vein stenting alone, with authors recommending that iliac vein stenting alone should be the primary treatment, with staged OVE to be reserved only in cases of persistent symptoms.5
Long-term effects of ovarian vein embolization and
iliac vein stenting for women of reproductive age
and anticoagulation management
iliac vein stenting for women of reproductive age
and anticoagulation management
OVE seems to be safe in patients of reproductive age and attempting to become pregnant.38,39 In a small series, subsequent pregnancies were reported in up to 66.7% of women undergoing OVE who had complete symptomatic relief.38 However, pregnancy is associated with recurrent reflux in the pelvic veins in women who had previously been treated with coil embolization, and a repeat procedure may be needed afterwards.39
On the other hand, there have been questions about long term patency of stents during pregnancy, and the decision of placing an iliac venous stent in the PeVD woman of childbearing age remains controversial, particularly in cases of isolated S2 symptoms.36 To that end, initial studies recommended perinatal anticoagulation management40; however, most recently, a systematic review demonstrated that in the heterogenous patient population with a subset treated for nonthrombotic iliac stenosis (46%), the following outcomes were recorded: 1.14% experienced stent occlusion, 2.29% developed asymptomatic nonocclusive in-stent thrombus, and 2.29% experienced permanent stent compression.41
There is marked variation in anticoagulation and antiplatelet management after stenting for nonthrombotic central venous stenosis. A Delphi consensus was performed to generate consensus statements among venous stenting experts. They stated that anticoagulation is preferred to antiplatelet therapy for the first 6 to 12 months after stenting for nonthrombotic venous stenosis. Low-molecular weight heparin is the first-choice anticoagulation the first 2 to 6 weeks after stenting. In the case of postthrombotic venous stenting, anticoagulation can be discontinued 6 to 12 months after stent placements if the following criteria are met: i) negative results are obtained on thrombophilia screen; ii) the thrombotic event was the first for the patient; and iii) stent patency is demonstrated on ultrasound. In patients who have multiple deep venous thromboses and iliac vein stenting, anticoagulation should be continued indefinitely barring contraindications.42
In our practice, patients who are in a hypercoagulable state or have a history of prior deep venous thrombosis are placed on anticoagulation for 6 months only. All other patients are treated on an antiplatelet agent (clopidogrel 75 mg) for 6 to 8 weeks after the procedure. Our rationale is that, in general, our patient population is young and healthy and presents with nonthrombotic disease. The risks of both bleeding and thrombosis are discussed with patients prior to making the decision of using antiplatelets versus anticoagulation.
Future trials considerations
The major challenge in the interpretation of results reported in the literature relates to heterogenous patient cohorts and to nonstandard terminology in describing the anatomical lesions and patient symptoms. Given the overlapping presentations and heterogeneity of clinical studies published thus far, it is prudent to optimize treatment using proper categorization of patients and data on existing literature. In terms of patients who present with IVS, most studies are based on patients who have associated chronic venous disease, predominantly manifested as VO-CPP. Daugherty et al29 demonstrated successful clinical outcomes in patients with PeVD and iliac vein stenting alone, whereas Gavrilov et al30 found that stenting alone was not sufficient to completely improve symptoms in a patient population with VO-CPP and S2,3 symptoms.
For future trials, we need first to study patients with S2 symptoms in a randomized controlled study with a comparator treatment. Whereas patients with OVR and IVS, would need to be evaluated separately, to understand the value of OVE and iliac vein stenting, a prospective evaluation of patients with both reflux and stenosis with S2 symptoms alone would be helpful to elucidate the order of treatments. Diagnostic criteria, as it relates to defining a clinically significant IVS threshold, needs to be further investigated to determine which lesions should be treated. At this point, the SVP classification has been designed as a new tool for defining the anatomy and symptoms of patients with PeVD. Prospective registries applying the SVP tool will help to evaluate treatment outcomes and understand the incidence and contribution of reflux versus obstruction. Long term data would then include controlled, prospective analysis of different treatment strategies and comparative studies to support interventional treatments in these patients. Actually, the lack of validated patient-reported–outcome tools limits the evaluation of the clinical benefit of treatment, and most studies rely on patients’ self-reported levels of pain as a clinical outcome metric.
Conclusion
Whereas there are different anatomical presentations, the management of combined patterns in PeVD has gained increased attention, with considerable variation in the literature in terms of patient selection, treatment order, and reported clinical outcomes. Although we are still debating the proper pathophysiology in these patients, and given the lack of high-quality scientific evidence, staged OVE followed by stenting seems to be a reasonable approach, particularly in patients with significant venous stenosis. However, there are still concerns about long-term outcomes in younger patients, particularly in women of childbearing age. Future research should focus on individual trials evaluating patients with isolated OVR, IVS, and S2 symptoms separately. This would then follow with the development of protocols to further elucidate the hemodynamic differences in patients with combined patterns. Ultimately, this would help define the appropriate criteria to choose therapy for the presenting clinical picture and underlying pathophysiology.

strong>CORRESPONDING AUTHOR
Gloria Salazar, MD, FSIR, FCIRSE
101 Manning Drive, Campus Box #7510,
Chapel Hill, North Carolina 27615,
United States
EMAIL: gloria_salazar@med.unc.edu
References
1. Khilnani NM, Meissner MH, Learman LA, et al. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30(6):781-789.
2. Meissner MH, Khilnani NM, Labropoulos N, et al. The Symptoms-Varices Pathophysiology classification of pelvic venous disorders: a report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord. 2021;9(3):568-584.
3. Smith SJ, Sichlau MJ, Smith BH, Knight DR, Chen B, Rowe PC. Improvement in chronic pelvic pain, orthostatic intolerance and interstitial cystitis symptoms after treatment of pelvic vein insufficiency. Phlebology. 2024;39(3):202-213.
4. Tanaka ME, Kutsenko O, Salazar G. Choosing the most appropriate treatment option for pelvic venous disease: stenting versus embolization. Semin Intervent Radiol. 2021;38(2):182-188.
5. Lakhanpal G, Kennedy R, Lakhanpal S, Sulakvelidze L, Pappas PJ. Pelvic venous insufficiency secondary to iliac vein stenosis and ovarian vein reflux treated with iliac vein stenting alone. J Vasc Surg Venous Lymphat Disord. 2021;9(5):1193- 1198.
6. Sulakvelidze L, Tran M, Kennedy R, Lakhanpal S, Pappas PJ. Presentation patterns in women with pelvic venous disorders differ based on age of presentation. Phlebology. 2021;36(2):135-144.
7. Szaflarski D, Sosner E, French TD, et al. Evaluating the frequency and severity of ovarian venous congestion on adult computed tomography. Abdom Radiol (NY). 2019;44(1):259-263.
8. Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES. Incompetent and dilated ovarian veins. AJR Am J Roentgenol. 2001;176(1):119-122.
9. Larkin TA, Hovav O, Dwight K, Villalba L. Common iliac vein obstruction in a symptomatic population is associated with previous deep venous thrombosis, and with chronic pelvic pain in females. J Vasc Surg Venous Lymphat Disord. 2020;8(6):961-969.
10. Santoshi RKN, Lakhanpal S, Satwah V, Lakhanpal G, Malone M, Pappas PJ. Iliac vein stenosis is an underdiagnosed cause of pelvic venous insufficiency. J Vasc Surg Venous Lymphat Disord. 2018;6(2):202- 211.
11. Possover M, Khazali S, Fazel A. Pelvic congestion syndrome and May-Thurner syndrome as causes for chronic pelvic pain syndrome: neuropelveological diagnosis and corresponding therapeutic options. Facts Views Vis Obgyn. 2021;13(2):141-148.
12. Knuttinen M-G, Zurcher KS, Khurana N, et al. Imaging findings of pelvic venous insufficiency in patients with postural orthostatic tachycardia syndrome. Phlebology. 2021;36(1):32-37.
13. Eklof B, Perrin M, Delis KT, Rutherford RB, Gloviczki P; American Venous Forum; European Venous Forum; International Union of Phlebology; American College of Phlebology; International Union of Angiology. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(02):498- 501.
14. Chait J, Sen I, Kalra M. Nutcracker syndrome: how to diagnose it and when/ how should it be treated in the pelvic venous disease population. Tech Vasc Interv Radiol. 2021;24(1):100734.
15. Maratto S, Khilnani NM, Winokur RS. Clinical presentation, patient assessment, anatomy, pathophysiology, and imaging of pelvic venous disease. Semin Intervent Radiol. 2021;38(2):233-238.
16. Garcia-Jimenez R, Valero I, Borrero C, et al. Transvaginal ultrasonography predictive model for the detection of pelvic congestion syndrome. Quant Imaging Med Surg. 2023;13(6):3735-3746.
17. Labropoulos N, Jasinski PT, Adrahtas D, Gasparis AP, Meissner MH. A standardized ultrasound approach to pelvic congestion syndrome. Phlebology. 2017;32(09):608- 619.
18. Eliahou R, Sosna J, Bloom AI. Between a rock and a hard place: clinical and imaging features of vascular compression syndromes. Radiographics. 2012;32:E33-E49.
19. Kuo YS, Chen CJ, Chen JJ, et al. May-Thurner syndrome: correlation between digital subtraction and computed tomography venography. J Formos Med Assoc. 2015;114(4):363-368.
20. Metzger PB, Rossi FH, Kambara AM, et al. Criteria for detecting significant chronic iliac venous obstructions with duplex ultrasound. J Vasc Surg Venous Lymphat Disord. 2016;4(1):18-27.
21. Kibbe MR, Ujiki M, Goodwin AL, et al. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(5):937-943.
22. Froehlich JB, Prince MR, Greenfield LJ, et al: “Bull’s-eye” sign on gadoflinium enhanced magnetic resonance venography determines thrombus presence and age: a preliminary study. J Vasc Surg. 1997;26:809e16.
23. Behzadi AH, Khilnani NM, Zhang W, et al. Pelvic cardiovascular magnetic resonance venography: venous changes with patient position and hydration status. J Cardiovasc Magn Reson. 2019;21:3.
24. Antignani PL, Lazarashvili Z, Monedero JL, et al. Diagnosis and treatment of pelvic congestion syndrome: UIP consensus document. Int Angiol. 2019;38(4):265- 283.
25. Beard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2:946-949.
26. Secemsky EA, Aronow HD, Kwolek CJ, et al. Intravascular ultrasound use in peripheral arterial and deep venous interventions: multidisciplinary expert opinion from SCAI/AVF/AVLS/SIR/SVM/SVS. J Vasc Interv Radiol. 2024;35(3):335-348.
27. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6(1):48- 56.e1.
28. Thorpe PE. Identification and treatment of restenosis in failing venous stents: the role of intravascular ultrasound. J Vasc Surg Venous Lymphat Disord. 2014;2(1):109- 110.
29. Daugherty SF, Gillespie DL. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2015;3(3):283-289.
30. Gavrilov SG, Vasilyev AV, Krasavin GV, Moskalenko YP, Mishakina NY. Endovascular interventions in the treatment of pelvic congestion syndrome caused by May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2020;8(6):1049-1057.
31. Daniels JP, Champaneria R, Shah L, Gupta JK, Birch J, Moss JG. Effectiveness of embolization or sclerotherapy of pelvic veins for reducing chronic pelvic pain: a systematic review. J Vasc Interv Radiol. 2016;27(10):1478-1486.e8
32. Sutanto SA, Tan M, Onida S, Davies AH. A systematic review on isolated coil embolization for pelvic venous reflux. J Vasc Surg Venous Lymphat Disord. 2022;10(1):224-232.e9.
33. Neuenschwander J, Sebastian T, Barco S, Spirk D, Kucher N. A novel management strategy for treatment of pelvic venous disorders utilizing a clinical screening score and non-invasive imaging. Vasa. 2022;51(3):182-189.
34. Sayed MH, Salem M, Desai KR, O’Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord. 2022;10:482-490.
35. Snow C, Pappas S, Sulakvelidze L, Kennedy R, Lakhanpal S, Pappas PJ. Nitinol stents placed in iliac veins are not associated with prolonged back pain. Phlebology. 2023;38:44-50.
36. Kutsenko O, McColgan Y, Salazar G. Iliac Vein stenosis: is the data strong enough for stenting in the young pelvic venous disorders (PeVD) population? Tech Vasc Interv Radiol. 2021;24(1):100733.
37. Esposito A, Charisis N, Kantarovsky A, Uhl JF, Labropoulos N. A comprehensive review of the pathophysiology and clinical importance of iliac vein obstruction. Eur J Vasc Endovasc Surg. 2020;60(1):118- 125.
38. Liu J, Han L, Han X. The effect of a subsequent pregnancy after ovarian vein embolization in patients with infertility caused by pelvic congestion syndrome. Acad Radiol. 2019;26(10):1373-1377.
39. Dos Santos SJ, Holdstock JM, Harrison CC, Whiteley MS. The effect of a subsequent pregnancy after transjugular coil embolisation for pelvic vein reflux. Phlebology. 2017;32(1):27-33.
40. Hartung O, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Management of pregnancy in women with previous left ilio-caval stenting. J Vasc Surg. 2009;50(2):355- 359.
41. Villalba L, Vaddavalli VV, Tripathi RK. Iliac vein stenting and pregnancy. J Vasc Surg Venous Lymphat Disord. 2023;11(6):1276-1284.
42. Milinis K, Thapar A, Shalhoub J, Davies AH. Antithrombotic therapy following venous stenting: international Delphi consensus. Eur J Vasc Endovasc Surg. 2018;55(4):537-544.