Local treatment of venous leg ulcers
Hôpital Tenon
Paris, France
ABSTRACT
The current standard of care for chronic venous ulcers involves the use of compression bandages. Dressings are applied beneath the compression and are used to control the exudates and to maintain the wound in a moist environment. Modern dressings are occlusive or semi-occlusive, classified according to their physical composition. Published systematic reviews of the value of different types of dressings in the management of chronic wounds provide only weak levels of evidence of their clinical efficacy, in terms of healing rate. Nevertheless, the indications for modern dressings were recently determined according to a systematic review of the literature and to a formal consensus process. Despite the lack of appropriate studies, modern dressings remain a part of the standard of care and are widely used according to the experience of the clinicians, in larger indications than what may be recommended by evidence-based medicine.
Skin grafting should be considered for large or refractory ulcers, when the venous hypertension is well controlled and when the ulcer bed is clean with healthy granulation tissue.
Topical negative pressure seems to prepare chronic wounds more rapidly for secondary closure surgery, but its clinical value in venous leg ulcers is still debated. More recently, local alternative treatments such as biological dressings and tissue-engineered products have been developed. These products may have the property of interacting directly with the wound, in order to speed the healing process and decrease the time to complete healing. But there is not yet any clear evidence for the efficacy of most of them.
INTRODUCTION
Venous ulcers are characterized by a cyclical pattern of healing and recurrence. The current standard of care for chronic venous ulcers involves the use of compression bandages as a means to reduce ambulatory venous pressure, control edema, and improve venous return. Dressings are applied beneath the compression and are used to control the exudates and to maintain the wound in a moist environment. Since the 1960s it has been accepted that wound healing is optimal when the wound is kept in a moist environment rather than air dried.1 Modern dressings are occlusive or semiocclusive, classified according to their physical composition. They have been developed to reduce pain and healing time, absorb blood and exudates and to be painless on application and removal. Current clinical practice guidelines on the treatment of leg ulcers have not established a consensual local care strategy, as published systematic reviews of the value of different types of dressings in the management of chronic wounds provide only weak levels of evidence of their clinical efficacy.2-4 Thus, the choice of the dressing is mainly based on clinical experience and on their absorbent capacity, hydrating ability, adhesive components, and debridement capacity. In fact, except for hydrocolloids, no significant difference has been demonstrated versus the reference treatment, which consists in ensuring a moist environment for the wound through the use of gauze soaked in physiological saline.2-6 Modern dressings optimize the natural healing process, without accelerating it. They mainly improve the comfort and quality of life of patients and reduce the cost of care by allowing reduced frequency of dressing changes.
More recently, local alternative treatments such as topical growth factors, biological dressings, and tissueengineered products have been developed. These products may have the property of interacting directly with the wound, in order to speed the healing process and decrease the time to complete healing. Most of these treatments are expensive, which may limit their widespread use, and there is not yet any clear evidence for the efficacy of most of them.
PRINCIPLES OF WOUND CARE
Moisture and occlusion
In the 1960s, Winter demonstrated that acute wounds covered with moisture-retentive occlusive dressings healed twice as rapidly as similar wounds left exposed to air. In contrast, excessively dry wound healing environments caused further tissue death. Thus, modern wound dressings have evolved from the older concept of leaving the wound dry and covered by a protective dressing to the new concept of protection of the wound environment.7 Semi-occlusive or occlusive wound dressings prevent evaporative water loss from the wound and retain warmth, which improves wound healing. These dressings may also induce relative hypoxia at the wound surface, promoting keratinocyte motility and angiogenesis.8
Because leg ulcers are invariably colonized by bacteria, infectious complications seem likely to be more prevalent with the use of occlusive dressings. In fact, infection rates are lower with occlusive dressings than with nonocclusive dressings, probably because they have the ability to maintain a more effective barrier against external contamination.9,10 Nevertheless, when the wound is clinically infected, with increased erythema, warmth, pain and exudates, absorbent dressings such as alginates are used rather than occlusive dressings such as hydrocolloids.
Antiseptics and antibiotics
It has been suggested recently that bacterial density is associated with the probability of nonhealing in leg ulcers when infection is detected using swabs or tissue biopsies, and that chronic wound healing may also be influenced by the diversity of microorganisms present and their interactions with one another.11 On the other hand, antiseptics and antibiotics fail to promote the healing process and to reduce the bacterial density of the wound.12,13 A recent Cochrane review confirms this, as there is actually no evidence to support the routine use of systemic antibiotics to promote healing in venous leg ulcers and the available evidence of topical antibiotic and antiseptic efficacy is not strong.14 In fact, the cytotoxic effects of antiseptics on pivotal cell types of the healing process have been well documented. Moreover, topical antibiotics and antiseptics are responsible for a great proportion of contact dermatitis in patients with leg ulcers,15-17 and the use of topical antibiotics may induce the emergence of organisms resistant to the entire class of the antibiotic used topically. In conclusion, antiseptic solutions for cleansing the wound are now avoided in routine care of chronic wounds and leg ulcers.7,18 Leg ulcers are cleaned using gentle soap and water. Therefore, guidelines recommend that systemic antibiotics should be reserved only for clinically infected ulcers and not for bacterial colonization.7
Debridement
Removal of necrotic tissue and slough is thought to allow formation of good granulation tissue and to promote epithelialization. Therefore, any necrotic material should be cleared from the wound bed to allow wound healing to proceed correctly. Wound bed preparation is now recognized as crucial to facilitating ordered restoration and regeneration of damaged tissue. However, there is a clear lack of good clinical evidence to support available wound debridement options, particularly for chronic ulcers of the lower extremities.
Mechanical debridement may be accomplished with a curette, scissors or a scalpel, or with hydrosurgery such as Versajet™, a new technology that simultaneously cuts and aspirates soft tissue. Mechanical debridement is a rapid and selective method, as nonviable tissue is removed until well vascularized tissue appears. However, the procedure may be painful, although Emla® cream has been shown to provide effective pain relief when applied 30 minutes before the procedure.19 Autolytic debridement is the progressive separation of slough and necrotic tissue from the wound bed, obtained by dressings that keep the wound in a moist environment. It may take several weeks but is painless and often used in association with mechanical debridement. Chemical debridement is obtained by using enzyme-debriding agents. Several topical enzymatic preparations are available in different countries, including collagenase, papain, and trypsin. A double-blind, randomized study showed that Elase™, the only enzymatic agent available in France, was ineffective in debriding venous ulcers.20
Maggot debridement is generally a safe therapy that removes sloughy necrotic tissue from ulcers and may eliminate methicillin-resistant Staphylococcus aureus from infected or colonized wounds.21,22 Bagged larval therapy seems to be well tolerated by patients, but is currently available in only a few hospitals in France.
DRESSINGS
Indications for the dressings
Although topical treatment is an important aspect of wound care, it should always be considered secondary to the choice of a compression strategy. Generally, the choice of dressing is guided by the ulcer characteristics (for example, wound drainage absorption), patient requirements (ease of application, comfort), and expense. According to recent systematic reviews, there is little evidence to indicate which dressings are the most effective in chronic wound care,2,4,5,23 because of the poor methodological quality of most studies of wound dressings. The Haute Autorité de Santé in France has determined the indications for modern dressings, according to a systematic review of the literature and to a formal consensus process2,24 (see Table I). Despite a lack of appropriate studies, modern dressings remain part of standard care and are widely used according to the experience of the clinicians, in more indications (Table II) than recommended by the Haute Autorité de Santé.
Different types of dressings (Table II)
HYDROCOLLOIDS
The inner layer of all hydrocolloids is composed of carboxymethyl cellulose, enclosed in an elastic adhesive mass. Hydrocolloids are available in thick and thin versions, as paste to fill cavity wounds, and in a variety of pre-cut shapes aimed at different anatomical sites (heels, sacrum, elbows). The rate of dressing changes is between a few days and a week, depending on the amount of exudate. As it interacts with the exudate, the dressing forms a yellow gel with a characteristic foul smell that can be mistaken for purulent discharge from the wound. An erythematous eruption around the wound is usually a nonallergic irritant reaction, related to excessively frequent dressing changes. They can be used at all stages of healing. The film covering the sheet protects the wound from the outside and allows patients to take a shower.6
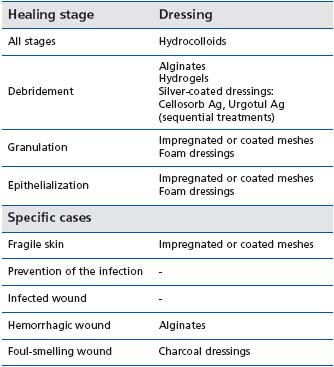
Table I: Indications of the dressings for chronic wounds, according
to the Haute Autorité de Santé in France2,24
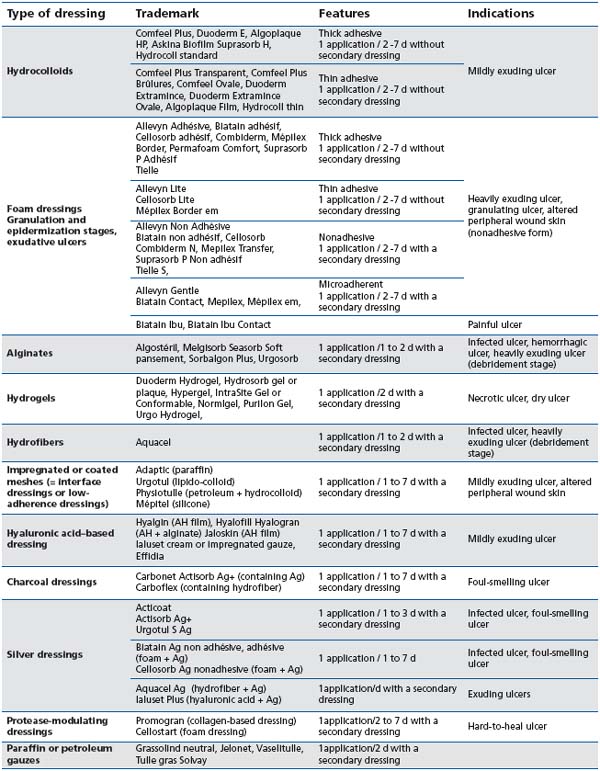
Table II : Different types of dressings and their common indications
HYDROGELS
Hydrogels are insoluble cross-linked hydrophilic polymers, containing more than 80% water. They are available in an amorphous gel, packaged in tubes, or in sheet form. The gel form appears to be the most effective in releasing moisture into wounds. A secondary dressing is necessary, such as a hydrocolloid or a polyurethane film. The dressing is changed every 3 or 4 days. Hydrogels are indicated for dry wounds, at the debridement stage. They are amongst the most efficient products in softening a necrotic plaque. Hydrogels may induce an allergic reaction around the wound, related to the presence of propylene glycol in some products,16-18 justifying the temporary use of them, at the debridement stage of the leg ulcer.
POLYURETHANE FILMS
Transparent film dressings are made of a polyurethane membrane coated on one side with an adhesive. They are permeable to gases and moisture vapor, but impermeable to water and bacteria. They have no absorbent capacity.
Films are indicated in superficial poorly exudating wounds such as skin tears, low-grade pressure ulcers, and at the epidermization stage of a wound, but are mostly used as a secondary dressing to hold another dressing in place.
ALGINATES
These polymers are mainly composed of fibers of calcium alginate derived from seaweed. They are sometimes mixed with carboxymethylcellulose, in varying percentages. They are commercially available in the form of sheets or ropes for cavities. They need to be covered with a secondary dressing (such as a polyurethane film, or gauze). They have a high absorbent capacity, and a mild bacteriostatic and hemostatic effect. They are indicated for heavily exuding wounds, and infected or hemorrhagic wounds, mainly at the debridement stage.25 The dressing is changed daily during the cleansing phase, every two or three days during granulation.
HYDROFIBERS
This dressing is made of carboxymethylcellulose fibers and presented in the form of sheets or ropes. The absorbent capacity is almost 2 or 3 times that of alginates. It can be used like an alginate, on heavily exuding wounds, and has to be covered with a secondary dressing. Under a hydrocolloid sheet, it can usually be changed every 3 or 5 days. On the surface of a wound, it interacts with exudate to form a cohesive gel, so hydrofiber dressings do not adhere to the wound. Hydrofibers are proposed at the wound debridement stage.
FOAM DRESSINGS
Foam dressings are usually made of a hydrophilic layer (microporous polyurethane) combined with a film as outer layer. They are available in adhesive and nonadhesive forms as well as in thick or extra-thin versions. Hydroabsorbent or superabsorbent dressings are similar to foam dressings, and come from the diaper industry. Foam dressings are highly absorbent and do not disintegrate in the wound, thus preventing the odors that may be experienced with hydrocolloids. In their nonadhesive form, they can be used even if the skin around the wound is irritated or macerated. The rate of dressing changes ranges from 3 to 8 days. They are indicated particularly from the granulation stage to complete closure for exuding chronic wounds. One of them (Biatain Ibu™) is impregnated with ibuprofen in order to provide local pain relief. sup>26
CHARCOAL DRESSINGS
These dressings contain a layer of charcoal, combined with an absorbent dressing. Active charcoal absorbs odors from the wound, which are infected or colonized by anaerobic or Gram-negative bacteria. These dressings can be moistened with physiological saline. They need to be covered with a secondary dressing. They are indicated as a primary or secondary dressing for infected wounds and for cancerous wounds.27 Some of them contain silver salts that are supposed to have an antiinflammatory effect or to decrease the bacterial load of the wound.
SILVER-COATED DRESSINGS
Silver acts as a broad-spectrum antibacterial agent. Silver dressings are widely employed for the treatment of infected wounds or chronic wounds with a high risk of infection as recent clinical studies suggest that the probability of chronic wounds healing properly is limited when the bacterial load is high.11 Unlike acute wounds and burns, the clinical benefit of a reduction in wound bacterial colonization is not established in chronic wounds. Most of the products also contain other components such as hydrocolloid, hyaluronic acid, alginate, or foam. A recent meta-analysis indicates that there is insufficient evidence to recommend the use of silver-containing dressings.28 Since this meta-analysis, a randomized controlled trial has shown that a 4-week treatment with a silver-releasing lipido-colloid contact layer increases significantly at 4 and 8 weeks the mean area reduction of venous leg ulcers with inflammatory signs that suggest a high bacterial load.29
IMPREGNATED OR COATED MESHES
(also called “lowadherence dressings” or “interface dressings”) Impregnated or coated meshes, which are less adherent and have a tighter mesh, thereby avoiding traumatic and hemorrhagic removal of the dressing, have now mainly replaced classic paraffin or petroleum gauzes. More recently designed impregnated or coated meshes are impregnated with hypoallergenic, neutral substances such as petroleum, paraffin, silicone, carboxy – methylcellulose, or lipido-colloid particles. These interface dressings do not adhere to the wound and need to be covered with a secondary, absorbent dressing. They are changed between once a day and twice a week. They are indicated for slightly exuding wounds, or chronic wounds, whatever the stage of the wound, especially when the peripheral wound skin is altered.
HYALURONIC ACID–BASED DRESSINGS
The rationale for the use of hyaluronic acid or collagen is to promote healing because they are present at a very high level in the dermis. Cream, impregnated tulles or dressings containing hyaluronic acid, sometimes in combination with alginates, are available. They have to be changed daily and this may be costly. They are used for mildly exuding chronic wounds at the stage of granulation, but may induce a burning sensation.
PROTEASE MODULATING DRESSINGS
Two such dressings are commercially available: Promogran™, which is composed of collagen and oxidized regenerated cellulose, and Cellostart™, which is a foam dressing where a nano-oligosaccharide factor is incorporated.
These dressings are supposed to reduce the protease activity of the fluids and to protect host growth factors against degradation. They are used on hard-to-heal wounds but are ineffective for infected wounds or unhealthy wound beds. Only Cellostart™ is reimbursed in France. A recent comparative study of these 2 products showed a significant reduction of the mean wound area in the Cellostart™ group compared with the Promogran group at 12 weeks of treatment.30
SKIN GRAFTS AND EMERGING BIOLOGICAL
TREATMENTS
Whilst compression therapy treats the underlying pathology, ulcers remain open in some cases for months or years, or heal very slowly. Additional treatments such as skin grafts or tissue-engineered skin may be used to hasten the healing process.
Skin grafts used for venous leg ulcers are most commonly pinch grafts, but split-thickness skin meshed grafts may also be performed on larger wounds. There are no specific indications for when skin grafting for venous leg ulcers should be used, but grafting should be considered for large or refractory ulcers, when the venous hypertension is well controlled and when the ulcer bed is clean with healthy granulation tissue.7,15 Despite the common use of skin grafts in venous leg ulcers, no valuable study is available to assess and quantify the effect of grafting on the healing of venous ulcers31 and to compare this strategy of treatment with other strategies, such as standard wound care.
Apligraf™ is a living bi-layered bioengineered skin substitute. It is composed of a type I collagen matrix in which human foreskin–derived neonatal fibroblasts are grown, and over which human foreskin–derived neonatal keratinocytes are then cultured and allowed to stratify. It was approved by the FDA in 1998 for the treatment of leg ulcers of greater than one-month duration that have not adequately responded to conventional therapy. Used with compression, Apligraf™ heals venous leg ulcers more effectively than simple dressings and compression, from 49% of complete closure to 63% at 6 months.31,32 Therefore, Apligraf™ is expensive, which limits its use, and is still not available in Europe.
An autologous keratinocyte suspension in a fibrin sealant matrix was recently compared with standard care in the healing of recalcitrant venous leg ulcers in a randomized controlled study. The group treated by cell therapy achieved complete healing in 38.3% of cases compared with 22.4% in the control group, and time to complete healing was significantly reduced by the cell therapy.33 Oasis™ is a biomaterial obtained from porcine smallintestine submucosa. It consists primarily of a collagen-based extracellular matrix that contains glycoaminoglycans, proteoglycans, fibronectin, and growth factors. In a recent randomized clinical trial, after 12 weeks of treatment, 55% of the Oasis™-treated leg ulcers were healed, compared with 34% in the standardcare group.34
At this time, the efficacy of other emerging treatments such as topical recombinant growth factors or other products of tissue engineering is not sufficiently evident. Randomized controlled studies are lacking for many biological products.35
TOPICAL NEGATIVE PRESSURE
Topical negative pressure is used to promote healing of surgical wounds by using suction to drain excess fluid from wounds and to promote the formation of granulation tissue. Therapy involves first placing a foam or open-pored gauze dressing on a wound. A tube attached to a canister at one end and a suction device at the other is then inserted into the dressing and the area is sealed with a sticky film. The device delivers a controlled negative pressure of -50 to -125 mm Hg which can be applied constantly or intermittently.36 The first and best known variant is the Vacuum-Assisted Closure (VAC™). The treatment may speed up healing in patients with venous ulcers, given bed rest in hospital,37 but few such patients are likely to be treated in this way, because of cost. A recent Cochrane review38 indicates that published trials are insufficient to conclude that topical negative pressure significantly increases the healing rates of chronic wounds. Chronic wounds treated with topical negative pressure appear to be ready for secondary closure surgery (mainly grafts) between 1 and 10 days earlier than controls.36 As these chronic wounds takes months to heal, the clinical relevance of this difference is debated.

REFERENCES
1. Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738-746.
2. Chaby G, Senet P, Vaneau M, et al. Dressings for acute and chronic wounds: a systematic review. Arch Dermatol. 2007;143:1297-1304.
3. Palfreyman S, Nelson EA, Michaels JA. Dressings for venous leg ulcers: a systematic review and meta-analysis. Br Med J. 2007;335:244-256.
4. O’Donnell TF, Lau J. A systematic review of randomized controlled trials of wound dressings for chronic venous ulcer. J Vasc Surg. 2006;44:1118-1125.
5. Douglas WS, Simpson NB. Guidelines for the management of chronic venous leg ulceration. Report of a multidisciplinary workshop. Br J Dermatol. 1995;132:446-452.
6. Senet P, Meaume S. Les pansements hydrocolloïdes. Ann Dermatol Venereol. 1999;126:71-75.
7. Fonder MA, Lazarus GS, Cowan DA, Aronson-Cook B, Kohli AR, Mamelak AJ. Treating the chronic wound: a practical approach to the care of nonhealing wounds and wound care dressing. J Am Acad Dermatol. 2008;58:185-207.
8. O’Toole EA, Marinkovich MP, Peavay CL, Amieva MR, Furthmayr H, Mustoe TA. Hypoxia increases human keratinocyte mobility on connective tissue. J Invest Dermatol. 1997;100:2881- 2891.
9. Hutchinson JJ, Lawrence JC. Wound infection under occlusive dressings. J Hosp Infect. 1991;17:83-94.
10. Eaglstein WH. Moist wound healing with occlusive dressings: a clinical focus. Dermatol Surg. 2001;27:175-181.
11. Davies CE, Hill KE, Newcombe RG, et al. A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Rep Reg. 2007;15:17-22.
12. Alinivi A, Basissi P, Pini M. Systemic administration of antibiotics in the management of venous ulcers. A randomized clinical trial. J Am Acad Dermatol. 1986;15:186-191.
13. Hansson C, Faergemann J. The effect of antiseptic solutions on microorganisms in venous leg ulcers. Acta Derm Venereal. 1995;75:31-33.
14. O’Meara S, Al-Kurdi D, Ovington LG. Antibiotics and antiseptics for venous leg ulcers. Cochrane Database of Systematic Reviews 2008;CD003557.
15. Machet L, Couhé C, Perrinaud A, Hoarau C, Lorette G, Vaillant L. A high prevalence of sensitization still persists in leg ulcer patients: a retrospective series of 106 patients tested between 2001 and 2002 and a meta-analysis of 1975-2003 data. Br J Dermatol. 2004;150:929-935.
16. Saap L, Fahim S, Arsenault E, et al. Contact sensitivity in patients with leg ulcerations. A north American study. Arch Dermatol. 2004;140:1241-1246.
17. Reichert-Penetrat S, Barbaud A, Weber M, Schmutz JL. Ulcères de jambe. Explorations allergologiques dans 359 cas. Ann Dermatol Venereol. 1999;126:131-135.
18. Valancia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44:401-421.
19. Lok C, Paul C, Amblard P, et al. EMLA cream as a topical anesthetic for the repeated debridement of venous leg ulcers: a double blind, placebocontrolled study. J Am Acad Dermatol. 1999;40:208-213.
20. Falabella AF, Carson P, Eaglstein WH, Falanga V. The safety and efficacy of a proteolytic ointment in the treatment of chronic ulcers of the lower extremity. J Am Acad Dermatol. 1998;39:737-774.
21. Bowling FL, Salgami EV, Boulton AJM. Larval therapy: a novel treatment in eliminating methicillin-resistant staphylococcus aureus from diabetic foot ulcers. Diabetes Care. 2007;30:370- 371.
22. Steenvorde P, Jacob CE, VanDoorn L, Oskam J. Maggot debridement therapy of infected ulcers: patient and wound factors influencing outcome- a study of 101 patients with 117 wounds. Ann R Coll Surg Engl. 2007;89:596-602.
23. Vermeulen H, Ubbink DT, Goossens A, de Vos R, Legemate DA. Systematic review of dressings and topical agents for surgical wounds healing by secondary intention. Br J Surg. 2005;92:665-672.
24. Vaneau M, Chaby G, Guillot B, et al. Consensus panel recommendations for chronic and acute wound dressings. Arch Dermatol. 2007;143:1291-1294.
25. Belmin J, Meaume S, Rabus MT, Bohbot S. Sequential treatment with calcium alginate dressings and hydrocolloid dressings accelerates pressure ulcer healing in older subjects: a multicenter randomized trial of sequential versus nonsequential treatment with hydrocolloid dressings alone. J Am Geriatr Soc. 2002;50:269- 274.
26. Gottrup F, Jorgensen B, Karlsmark T, et al. Reducing wound pain in venous leg ulcers with Biatain-Ibu: a randomized, controlled double blind clinical investigation on the performance and safety. Wound Rep Regen. 2008;16:615-625.
27. Holloway S, Bale S, Harding K, Robinson B, Ballard K. Evaluating the effectiveness of a dressing for use in malodorous, exuding wounds. Ostomy Wound Manag. 2002;48:22-28.
28. Vermeulen H, Van Hattem JM, Storm- Versloot MN, Ubbink DT. Topical silver for treating infected wounds. Cochrane Database Syst Rev 2007;CD 005486.
29. Lazareth I, Meaume S, Sigal-Grinberg ML, et al. The role of a silver releasing lipido-colloid contact layer in venous leg ulcers presenting inflammatory signs suggesting heavy bacterial colonization: results of a randomized controlled study. Wounds. 2008;20:158- 166.
30. Schmutz JL, Meaume S, Fays S, et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: results of a randomised controlled study. Int Wound J. 2008;5:172-182.
31. Jones JE, Nelson EA. Skin grafting for venous leg ulcers. Cochrane Database Syst Rev 2007;CD 001737.
32. Falanga V, Margolis D, Alvarez O, et al. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Arch Dermatol. 1998;134:293-300.
33. Vanscheidt W, Ukat A, Horak V, et al. Treatment of recalcitrant venous leg ulcers with autologous keratinocytes in fibrin sealant a multinational randomized controlled clinical trial. Wound Rep Regen. 2007;15:308-315.
34. Mostow EN, Haraway D, Dalsing M, Hodde JP, King D, and the Oasis Venus Ulcer Group Study. Effectiveness of an extracellular matrix graft (Oasis wound matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005;41:837- 843.
35. Enoch S, Grey JE, Harding KG. ABC of wound healing. Recent advances and emerging treatments. Br Med J. 2006;332:962-965.
36. Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H. A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg. 2008;95:685-692.
37. Ubbink DT, Westerbos SJ, Evans D, Land L, Vermeulen H. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev 2008;CD001898.
38. Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (VAC) with modern wound dressings. J Vasc Surg. 2006;44:1029- 1038.
