Isolated calf deep vein thrombosis: to treat or not to treat
Andrew N. Nicolaides,
MS, DSc, PhD, FRCS
Vascular Screening and Diagnostic
Center, Nicosia, Cyprus; and
Department of Vascular Surgery,
Imperial College, London, United
Kingdom; and Department of Basic
and Clinical Science, University of
Nicosia Medical School, Nicosia,
Cyprus
ABSTRACT
On the basis that in the presence of isolated calf deep venous thrombosis (DVT) fatal pulmonary emboli (PE) did not occur while the patient was in hospital, there was one school of thought that routine anticoagulation was unnecessary and that ultrasound surveillance would suffice, reserving anticoagulation for those in whom the thrombus extends into the popliteal or more proximal veins. However, another school of thought, based on the realization that local damage to the venous valves with the development of reflux and skin changes, and symptoms of persistent pain and edema in 10% to 23% of patients leading to CEAP C4-C6 classes and a DVT recurrence rate of up to 14%, believed anticoagulation should be routine in such patients unless there were serious contraindications. Recent evidence from randomized controlled trials and meta-analyses indicates that isolated calf DVT should be treated. A key message from studies addressing the comparison between direct oral anticoagulants (DOACs) and conventional therapy for the initial and short-term therapy (3-6 months) is that treatment of acute isolated calf DVT with DOACs is as effective as standard therapy; also, it’s associated with a statistically significant reduction in the risk of major bleeding complications, clinically relevant as intracranial and fatal bleeding are the most reduced types.
Introduction
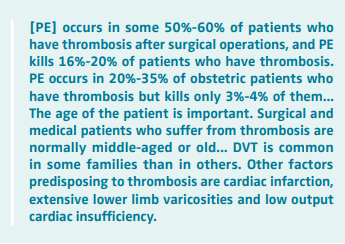
Relatively little was known about the true incidence of postoperative deep venous thrombosis (DVT) or its natural history prior to the 1960s, although by that time, it was established that the clinical diagnosis of DVT was unreliable because in the presence of symptoms and signs only 50% of the patients were found to have DVT on venography. In addition, leg veins were thought to be the common site for the origin of pulmonary emboli (PE).1 The available knowledge on DVT at that time had been summarized as follows by Ian Aird in his classic monograph “A Companion in Surgical Studies” published in 19582:
In 1960, Hobbs and Davies argued that the largest specific component of the thrombus was the fibrin network, and as fibrinogen could be labeled with radioactive iodine, it should be incorporated in a forming thrombus and detected by an external scintillation counter. They demonstrated the feasibility of this in rabbits using 131I.3,4
In 1965, Atkins and Hawkins5,6 working at Kings College Hospital Medical School, London, United Kingdom substituted the isotope 125I for 131I as the radioactive label because 125I had a longer half-life (60 days instead of 8 days) and a lower gamma radiation energy so that a lighter and more mobile apparatus could be used. The accuracy of the test was confirmed by venography,7-9 and by using a ratemeter9 it became a simple test suitable for the routine screening of a large numbers of patients.
True incidence of DVT
Several studies using the 125I-fibrinogen test demonstrated that patients who undergo surgical procedures are at high risk of developing venous thrombus embolism (VTE). The findings of these early studies were summarized in a review by Hobbs and Nicolaides in 1971.10 In the absence of prophylaxis, the risk of silent DVT was 30% in general surgery, 17% in gynecological surgery, 47% in patients with a fractured neck of femur, 30% in urological surgery, 34% in acute myocardial infarction, and 68% in medical patients in shock. It was found that the majority of the thrombi started during the operation and that 89% of thrombi started in the calf, 6.5% in the popliteal region, and 4% in the thigh.11 Subsequent venographic studies demonstrated that in patients with fractured neck of femur and elective hip replacement, calf DVT that occurred in the first week after surgery was often followed by iliac vein thrombosis, which tended to occur de novo in the second week after operation.12
Natural history of calf DVT
Of the thrombi that started in the calf, 20% lysed spontaneously. This was particularly so in patients who were ambulant. However, 25% of calf thrombi extended more proximally into the popliteal, femoral, or iliac veins. If the thrombosis was limited to the calf, the risk of serious PE during hospitalization was negligible, but if the popliteal and femoral veins became involved, the risk of PE rapidly increased, reaching 50% when the iliac veins were involved.13
During the 1970s, the 125I-fibrinogen test was used as a diagnostic tool in most randomized controlled trials (RCT) on the efficacy of different methods of prevention14 and was the tool used in RCT that established the value of low-dose heparin, low-molecular-weight heparin (LMWH), elastic compression, electrical calf muscle stimulation, and intermittent pneumatic compression in the prevention of postoperative DVT. These RCTs eventually demonstrated that if a method prevents calf DVT, it also prevents proximal DVT, symptomatic DVT, PE, and fatal PE.15,16 The 125I-fibrinogen test was eventually replaced by ultrasound, and with the appearance of AIDS in 1981 it became obsolete. However, its “legacy” and the problem of how to manage isolated calf DVT remained a controversial subject.
Two schools of thought
The finding that in the presence of isolated calf DVT fatal PE did not occur while the patient was in hospital resulted in a school of thought that routine anticoagulation was unnecessary and that surveillance with ultrasound would suffice, reserving anticoagulation for those in whom the thrombus extends into the popliteal or more proximal veins. However, the realization that local damage to the venous valves with the development of reflux and skin changes, and symptoms of persistent pain and edema in 10% to 23% of patients leading to CEAP (clinical-etiological-anatomical pathophysiological) C4-C6 classes and a DVT recurrence rate of up to 14%17-19 led to the development of another school of thought that such patients should be routinely anticoagulated unless there were serious contraindications.
DVT recurrence after isolated calf DVT
In 1984, a randomized study of 51 patients with symptomatic isolated calf DVT, of whom 23 received warfarin for 3 months and 28 did not, investigated the rate of recurrence.20 Recurrences and their extent were confirmed with venography. Both groups received an initial course of heparin, and all patients wore compression stockings. During the first 3 months, recurrence occurred in 29% of patients in the non-warfarin group compared with none in the warfarin group (P< 0.01). Five of these patients had a recurrence with proximal extension and 1 had a pulmonary embolus. At 1 year, 1 (4.3%) out of 23 patients in the warfarin group had a recurrence, compared with 19 (68%) out of 28 in the non-warfarin group (risk ratio [RR], 0.13; 95% CI, 0.02 to 0.99). The findings suggested that oral anticoagulants should be given to all patients with symptomatic isolated calf DVT and that 3 months seemed to be sufficient. However, a study in 2016 (the CACTUS study, [Contention Alone Versus Anticoagulation for Symptomatic Calf Vein Thrombosis Diagnosed by Ultrasonography]) questioned these conclusions.21 This was a double-blind, placebo-controlled RCT involving 259 low-risk outpatients (without active cancer or previous VTE) with a first acute symptomatic DVT in the calf who were assigned to receive either LMWH (nadroparin 171 UI/kg, subcutaneously, once daily) or placebo for 6 weeks. There was no significant difference between the groups in the composite primary outcome (a composite of extension of calf DVT to proximal veins, contralateral proximal DVT, and symptomatic PE), which occurred in 4 (3%) patients in the LMWH group and in 7 (5%) in the placebo group (P=0.54). Bleeding occurred in 5 patients (4%) in the LMWH group and no patients in the placebo group (P=0.03). It was concluded that nadroparin was not superior to placebo in reducing the risk of proximal extension or VTE events in low-risk outpatients with symptomatic calf DVT but did increase the risk of bleeding. Accordingly, in patients with acute isolated distal DVT of the leg and without severe symptoms or risk factors for extension, guidelines suggested serial imaging of the deep veins for 2 weeks over anticoagulation; by contrast, in patients with severe symptoms or risk factors for extension, anticoagulation was suggested over serial imaging of the deep veins.22
In the most recent double-blind RCT (RIDTS [Rivaroxaban for the treatment of symptomatic Isolated Distal deep vein Thrombosis]) addressing the optimal duration of anticoagulation in patients with symptomatic isolated calf DVT, the administration of therapeutic doses of rivaroxaban (20 mg once daily) for 3 months was found to reduce the incidence of recurrent VTE over a 2-year follow-up period compared with a shorter course (6 weeks) without increasing the hemorrhagic risk.23 Indeed, among the 404 patients that were recruited, the primary efficacy outcome (composite of isolated distal DVT, recurrent isolated distal DVT, proximal DVT, symptomatic PE, or fatal PE) occurred in 23 (11%) patients in the rivaroxaban arm and 39 (19%) in the placebo arm (RR, 0.59; 95% CI, 0.36 to 0.95). No major bleeding events occurred.
Systematic reviews and meta-analyses
A systematic literature review and meta-analysis of 24 studies on the duration of anticoagulant therapy in patients with isolated calf DVT, involving 2936 patients, was published in 2016.24 Of these, 5 studies were RCTs, 7 were prospective cohort studies, 7 were retrospective studies, and 1 was a combined prospective and retrospective cohort study. Four additional studies compared different durations of anticoagulation. Recurrent VTE (proximal propagation, recurrence of DVT or PE) was reduced from 11.1% in patients not on anticoagulation to 6.5% in patients on anticoagulation (odds ratio [OR], 0.50; 95% CI, 0.15 to 0.73) without increase in major bleeding. Recurrent DVT was reduced from 6.5% to 1.5% (OR, 0.23; 95% CI, 0.08 to 0.65), and PE was reduced from 2.4% to 1.4% (OR, 0.48; 95% CI, 0.25-0.91). The recurrence rate of VTE was reduced from 10.7% in those receiving anticoagulation for less than 6 weeks to 3.2% in those receiving anticoagulation for more than 6 weeks (OR, 0.39; 95% CI, 0.17 to 0.90). The authors concluded that in patients with isolated calf DVT, anticoagulation reduces the incidence of PE and recurrent DVT without increased risk of major bleeding. Although most patients on anticoagulants in the studies included in the meta-analysis were receiving vitamin K antagonists (VKA), the authors suggested that direct oral anticoagulants (DOACs) should be considered for treatment of isolated calf DVT, given their improved efficacy-to-safety profile.
The most recent Cochrane systematic review and meta analysis25 published in 2020 identified 8 RCTs involving 1239 patients with isolated calf DVT. In 5 trials, anticoagulation therapy was used up to 3 months; and in 3 trials, anticoagulation of different periods was used. Recurrence of VTE was reduced from 9.1% in the placebo/no intervention group to 2.9% in the VKA group (RR, 0.34; 95% CI, 0.15 to 0.77). There was no significant difference in the risk of PE, but the risk of DVT recurrence was reduced from 7.9% to 1.65% (RR, 0.25; 95% CI, 0.10 to 0.67). There was no significant increase in major bleeding, but there was an increase in clinically relevant non-major bleeding from 1.8% to 7.0% (RR, 3.34; 95% CI, 1.07 to 10.46). In 3 RCTs comparing treatment with VKA for 3 or more months with treatment for 6 weeks, treatment for 3 months or more reduced the incidence of VTE from 13.9% in the 6-week group to 5.8% in the 3-or more-months group (RR, 0.42; 95% CI, 0.26 to 0.68). The risk of recurrent DVT was also reduced from 14.4% to 4.8% (RR, 0.32; 95% CI, 0.16 to 0.64).
The end of the debate
The evidence from the RCTs and meta-analyses has now resulted in resolution of the debate on whether isolated calf DVT should be treated or not. These data indicate that isolated calf DVT should be treated.26-28 A key message from the above systematic reviews and meta-analyses addressing the comparison between DOACs and conventional therapy for the initial and short-term therapy (3-6 months) is the following: treatment of acute isolated calf DVT with DOACs is as effective as standard therapy and is associated with a reduction in the risk of major bleeding complications that is not only statistically significant but also clinically relevant, as intracranial and fatal bleeding are the most reduced types.24,25,29



CORRESPONDING AUTHOR
Professor Andrew Nicolaides
2 Kyriacou Matsi Street, Ayios
Dhometios, 2368 Nicosia, Cyprus
EMAIL: anicolaides1@gmail.com
References
1. Gibbs NM. Venous thrombosis of the lower limbs with particular reference to bedrest. Br J Surg. 1957;45:209-236.
2. Aird I. A Companion in Surgical Studies. E & S Livingstone Ltd; 1958:213.
3. Hobbs JT, Davies JWL. Detection of venous thrombosis with 131I-labelled fibrinogen in the rabbit. Lancet. 1960;2:134-135.
4. Hobbs JT. External measurement of fibrinogen uptake in experimental venous thrombosis and other local pathological states. Br J Exp Pathol. 1962;43:48-58.
5. Atkins P, Hawkins LA. Detection of venous thrombosis in the legs. Lancet. 1965;2:1217-1219.
6. Atkins P, Hawkins LA. The diagnosis of deep vein thrombosis in the leg using 125I-fibrinogen. Br J Surg. 1968;55(11):825-830.
7. Flanc C, Kakkar VV, Clarke MB. The detection of venous thrombosis in the leg using 125I-labelled fibrinogen. Br J Surg. 1968;55:742-747.
8. Negus D, Pinto DJ, Le Quesne LP, Brawn N, Chapman M. 125I-labelled fibrinogen in the diagnosis of deep vein thrombosis and its correlation with phlebography. Br J Surg. 1968;55:835-839.
9. Kakkar VV, Nicolaides AN, Renney JTG, Friend J, Clarke MB. 125I-labelled fibrinogen test adapted for routine screening for deep vein thrombosis. Lancet. 1970;1:540-542.
10. Hobbs JT, Nicolaides AN. The diagnosis of acute deep vein thrombosis with 125-Iodinated fibrinogen. In: Zeitler E, ed. Diagnostik mit Isotopenbei arteriellen und venösen Durchblutungsstörungen der Extremitäten. Hans Huber Publishers; 1971.
11. Nicolaides AN, Kakkar VV, Renney JTG. The origin of deep vein thrombosis: a venographic study. Br J Radiol. 1971;44:653-663.
12. Field ES, Nicolaides AN, Kakkar VV, Crellin RQ. Deep vein thrombosis in patients with fractures of the femoral neck. Br J Surg. 1972;59:377-379.
13. Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of deep vein thrombosis. Lancet. 1969;2:230-232.
14. Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism. Int Angiol. 2013;32(2)111-258.
15. Nicolaides AN, Desai S, Douglas JN, et al. Small doses of subcutaneous sodium heparin in preventing deep venous thrombosis after major surgery. Lancet. 1972;300:890-893.
16. Kakkar VV, Corrigan TP, Fossard DP, Sutherland I, Thirwel J. Prevention of fatal postoperative pulmonary embolism by low doses of heparin. Reappraisal of results of international multicentre trial. Lancet. 1977;1:567-569.
17. Masuda EM, Kessler DM, Kisner RL, Eklof B, Sato DT. The natural history of calf vein thrombosis: lysis of thrombi and development of reflux. J Vasc Surg. 1998;28:67-73.
18. Masuda EM, Kistner RL, Musikasinthorn C, Liquido F, Geling O, Qimei H. The controversy of managing calf vein thrombosis. J Vasc Surg. 2012;55:550- 561.
19. Saarinen JP, Domonui K, Zeitlin R, Salenius JP. Postthrombotic syndrome after isolated calf deep venous thrombosis: the role of popliteal reflux. J Vasc Surg. 2002;36:959- 964.
20. Langerstedt CI, Olsson CG, Fagher BO, Oqwist BW, Albrechtsson U. Need for long-term anticoagulant treatment in symptomatic calf-vein thrombosis. Lancet. 985;2:515-518.
21. Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016;3:e556-e562.
22. Stevens SM, Woller SC, Baumann Kreuziger L, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report – Executive Summary. Chest. 2021;160:2247-2259.
23. Ageno W, Bertù L, Bucherini E, et al. Six weeks versus 3 months of treatment with rivaroxaban for patients with symptomatic isolated distal deep vein thrombosis: a randomized controlled trial – RIDTS study. BMJ. 2023;379:e072623.
24. Franco L, Giustozzi M, Agnelli G, Becattini C. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta-analysis. J Thromb Haemost. 2016;15:1142-1154.
25. Kirkilesis G, Kakkos SK, Bicknell, Salim S, Kakavia K. Treatment of distal deep vein thrombosis. Cochrane Database Syst Rev. 2020;4(4):CD013422. doi:10.1002/14651858.CD013422.pub2
26. Nicolaides AN, Kakkos S. The “legacy” of the 125I-fibrinogen test and current management of isolated calf vein thrombosis: the end of a 40-year debate. Vasc Invest Ther. 2021;4(4):123-126.
27. Kakkos S, Gohel M, Baekgaard N, et al. Editor’s choice – European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61:9-82.
28. Nicolaides AN, Fareed J, Spyropoulos AC, et al. Prevention and management of venous thromboembolism. International Consensus Statement (Guidelines according to scientific evidence). Int Angiol. 2024;13(1)1-222.
29. van Es N, Coppens M, Schulman S, Middeldorp M, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124:1968-1975.

