Investigations in postthrombotic syndrome according to clinical status
INTRODUCTION
Many angiologists and vascular surgeons consider that postthrombotic syndrome (PTS) should only be managed noninterventional treatment. Unaware of the real possibilities provided by operational treatment, they limit investigation to color duplex scanning (CDS), which in this perspective is logical and reasonable. Compression, drugs, and lifestyle recommendations do not call for level III investigation according to the CEAP classification.1
Conservative treatment as far as etiology is concerned relies mainly on the signs and symptoms, independently of the location, extension of anatomical lesions, and pathophysiological abnormality. Conversely, when surgical or endovenous treatment is considered, the CEAP A and P descriptors must be defined precisely and the severity of PTS as a whole must be evaluated by accurate and informative investigations.
AIM OF THE ARTICLE
To provide guidelines for undertaking investigations according to the patient’s clinical presentation.
CHRONIC VENOUS INSUFFICIENCY INVESTIGATIONS
These investigations have been recently defined in special issues of the Journal of Vascular Surgery and International Angiology.2,3
1. Investigations providing morphological and anatomical information:
– Ultrasound investigations including color duplex scanning(CDS) and intravascular ultrasound (IVUS)
– Ascending and descending phlebography
– Spiral computed tomography – Magnetic resonance imaging
2. Investigations providing hemodynamic information and global information on chronic venous insufficiency (CVI) severity:
– CDS
– Pressure measurements: ambulatory venous pressure with and without tourniquet, arm/foot venous pressure differential, reactive hyperemia, foot venous pressure elevation, femoral vein pressure
– Plethysmography: photoplethysmography, air plethysmography (APG)
3. Investigations providing information on the microcirculation:
– They are not used in daily practice.
INDICATIONS FOR INVESTIGATIONS ACCORDING TO CLINICAL STATUS
1. In patients presenting PTS with few symptoms and without moderate or severe edema according to venous clinical score4 and/or skin changes, investigations can be limited to CDS. The same policy is recommended at follow-up visits when the patient is stable with or without compression.
2. When a patient compliant with suitable compression therapy is still symptomatic—persisting severe pain, venous claudication—or presents with severe edema, progressive worsening skin change, or recurrent ulcer, standard CDS should be completed by complementary investigations in the absence of severe systemic disease or when calf pump function cannot be improved (stiff ankle, calf muscular atrophy). The following investigations should be undertaken in order.
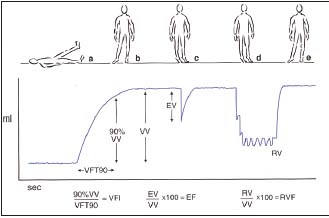
Figure 1. Air plethysmography
The different maneuvers and the parameters
VV= venous volume; VFT= venous filling time;
VFI= venous filling index
EV= ejected volume; EF= ejection fraction; RV= residual volume RVF= residual volume fraction
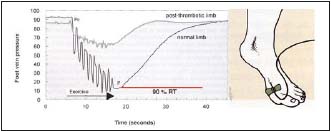
Figure 2. Ambulatory venous pressure measurement
A needle is inserted in a vein on the dorsum of the foot with the patient standing. Pressures are recorded before exercise and during a ten tiptoe exercise. The ambulatory venous pressure (AVP) is defined as the lowest pressure reached during the exercise.
P0= pressure before exercise; P= AVP; RT= refilling time
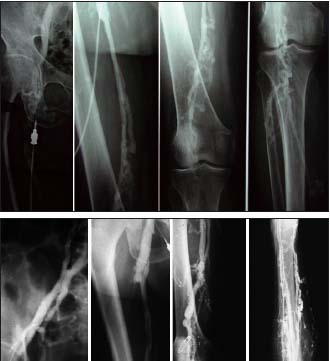
Figure 3 and 3b. Descending venography
Deep axial reflux grade 4 according to Kistner
Kistner, RL, Ferris RG, Randhawa G, Kamida CB. A method of performing descending venography. J Vasc Surg. 1986;4:464-468.
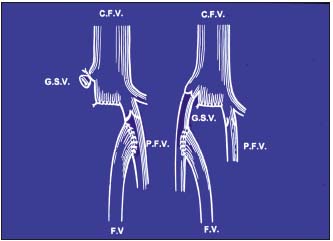
Figure 4. Femoral vein transposition
GSV = great saphenous vein; FV= femoral vein; CFV= common femoral vein
PFV= profunda femoris vein
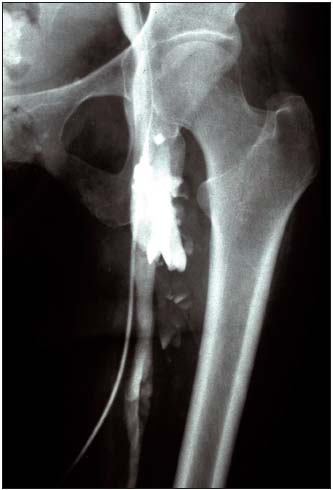
Figure 5. Descending venography with Valsalva maneuver
Incompetent femoral vein. Competent profunda femoris vein<
• To evaluate hemodynamics by means of air plethysmography (Figure 1) and ambulatory venous pressure (Figure 2), but neither provides information on anatomical disorders or pathophysiological mechanisms.
• When deep reflux is identified by CDS and ascending and descending venography is decided, ascending phlebography identifies the postthrombotic changes in the deep system and the collateral patterns. Descending venography determines, first, the extent of the reflux, which is crucial for determining whether deep venous reconstructive surgery is needed as only axial reflux has to be treated (Figures 3, 3b), and, second, what kind of surgery is feasible:
– Femoral vein transposition (Figure 4) to the great saphenous vein or profunda vein, insofar as its proximal valve is competent5 (Figure 5), knowing that valvuloplasty is rarely feasible in PTS
– If transposition is not feasible, phlebography combined with ultrasound scan provides information on the most suitable site for performing valve segment transplan tation6 (Figure 6) or neovalve construction7 (Figure 7).
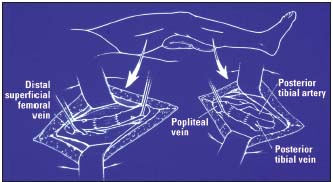
Figure 6. Valve segment transplantation
Transposition to the popliteal vein above and below the knee.
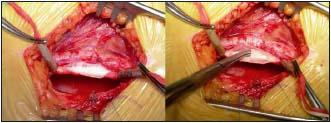
By Courtesy Prof Maleti (Italy)
Figure 7. Neovalve construction according to Maleti’s technique
Left: typical appearance of a postthrombotic vein after axial phlebotomy
Right: a monocuspid valve has been constructed with the thickened vein wall
• Quantification of venous obstruction is not easy. Traditional methods measure arm-foot pressure differential,8 outflow fraction,9 and outflow fraction resistance by plethysmography (Figure 8),10 but their specificity and sensitivity are inconsistent11,12 and they do not quantify local anatomic distribution.
• Iliocaval obstruction is not always identified by femoral vein pressure measurement and phlebography, as both can underestimate its severity.13 According to Neglen and Raju, intravenous ultrasound is more reliable (Figures 9-11),14,15 but is invasive and expensive.
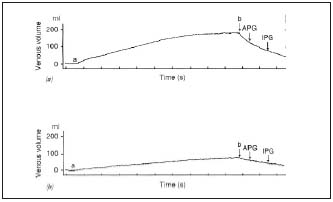
Figure 8. Plethysmography
Vein obstruction is determined through calf venous capacitance and maximal venous outflow, the patient supine with the limb elevated. A thigh cuff is inflated (a) to prevent venous outflow. The thigh cuff is deflated (b). Capacitance is determined as the volume difference b-a.
Maximal venous outflow is determined as the difference in volume at 1 s for air plethysmography or at 3 s for impedance plethysmography.
Above: normal subject
Below: patient with venous obstruction
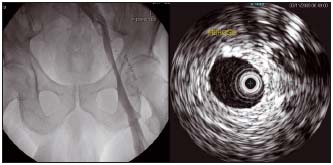
Figure 9. Ascending venography and intravascular ultrasound (same patient)
Extensive wall fibrosis is evident on intravascular ultrasound which will not be suspected from the corresponding venogram. (by courtesy Prof Raju, USA)
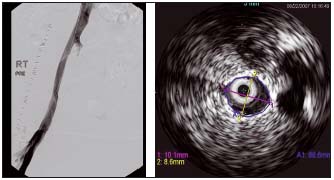
Figure 10. Ascending venography and intravascular ultrasound (same patient)
Another case of diffuse postthrombotic stenosis. The venogram looked normal, but intravascular ultrasound showed a stenosed external iliac vein 10 mm x 8 mm (normal 14 mm x 14 mm). (by courtesy Prof Raju, USA)
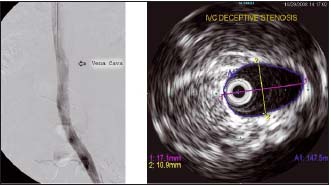
Figure 11. Phlebography and intravascular ultrasound of inferior vena cava (same patient)
This patient had stenosis of the inferior vena cava, which was not picked up on venography. The inferior vena cava looks normal, but on intravascular ultrasound its maximum diameter was only 17 mm. For an adult male, this should be around 23 mm. (by courtesy Prof Raju, USA)
The value of spiral computed tomography and magnetic resonance imaging is not yet clearly established.
• There is no reliable investigation for identifying infrainguinal obstructions since, as in iliocaval vein obstruction, phlebography is not reliable (Figure 12).
Some patterns need to be described:
– In severe PTS of the lower limb, the entire outflow seems to occur through the superficial system with nonvisualization of the deep system. This is a technical artifact despite the use of tourniquets.
– When the femoral vein is occluded, the profunda enlarges leading to axial transformation of the profunda femoris vein (Figure 13).16
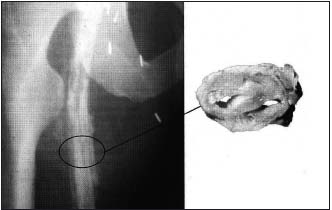
Figure 12. Left: Ascending venography
Intraluminal trabeculations are apparent, although the vein appears to be patent.
Right: Venous pathology. The vein lumen is reduced to 2 narrow channels.
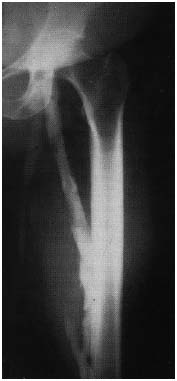
Figure 13. Ascending phlebography
Axial transformation of the profunda vein. (by courtesy Prof Raju, USA)
Fortunately, collateral formation seems to compensate better for femoro-popliteo-crural venous obstruction than for obstruction of the iliac and common femoral veins. Furthermore, there is no efficient treatment of infra-inguinal venous obstruction, although good results with endophlebectomy have been reported in a small series of patients.17
3. In PTS, obstruction and reflux are frequently associated and unfortunately there is no investigation that identifies the most important pathophysiological factor. Nevertheless, the consensus is to treat first iliocaval obstruction, when combined with below inguinal reflux, as the successful treatment of the former generally improves the latter.
CONCLUSION
Investigations in PTS must be tailored to the clinical situation and undertaken according to the planned treatment. Medical angiologists must realize that surgery and endovascular procedures can improve severe PTS not stabilized by conservative treatment.7,15 knowing that the key for to successful operative treatment is thorough investigation performed by a skilled and trained unit.

REFERENCES
2. Acute and chronic venous disease. J Vasc Surg. 2007;46(Suppl S):1-93.
3. Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. International Angiology. 2008;27:1-60.
4. Rutherford RB, Padberg FT Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000; 31:1307- 1312.
5. Kistner RL. Transpositions techniques in Atlas of venous surgery. JJ Bergan and RL Kistner (eds) WB Saunders 1992 Philadelphia:153-156.
6. Taheri SA, Heffner R, Bodd T, Pollack LH. Five years experience with vein valve transplant. World J Surg. 1986;10:935-937.
7. Lugli M, Guerzoni S, Garofalo M, Smedile G, Maleti O. Neovalve construction in deep venous incompetence. J Vasc Surg. 2009;49:156-162.
8. Raju S. New approaches to the diagnosis and treatment of venous obstruction. J Vasc Surg. 1986;4:42-54.
9. Kalodiki E, Calahoras LS, Delis KT, Zoukias CP, Nocolaides AN. Air plethysmography: the answer in detecting past deep venous thrombosis. J Vasc Surg. 2001;33:715-720.
10. Nicolaides AN. Investigation of chronic venous insufficiency. A consensus statement (France, March 5-9, 1977). Circulation. 2000;102:E126-163.
11. Labropoulos N, Volteas N, Leon M, et al. The role of venous outflow obstruction in patients with chronic venous obstruction. Arch Surg. 1977;132:46-51.
12. Neglen P, Raju S. Compliance of the normal and postthrombotic calf. J CardioVasc Surg. 1995;36:225-231.
13. Neglen P, Raju S. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583- 589.
14. Neglen P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694-700.
15. Neglen P. Chronic deep venous obstruction: definition, prevalence, diagnosis, management. Phlebology. 2008;23:149-157
16. Raju S, Fountani T, Neglen P, Devidas M. Axial transformation of the profunda femoris vein. J Vasc Surg. 1998;27:651-659.
17. Puggioni A, Kistner RL, Eklof B, Lurie F. Femoro-popliteal venous obstruction appears to be better compensated for by collateral formation than obstruction of the iliac and common femoral veins. J Vasc Surg. 2004;39:1048-1052.
