Invasive treatment of post-thrombotic symptoms
River Oaks Hospital, Flowood
Mississippi, USA
ABSTRACT
The treatment of symptomatic postthrombotic syndrome is a difficult, evolving, and lifelong undertaking. This chronic disease is not only characterized by the possibility of leg ulcer formation, but more often disabling pain and swelling with minimal skin changes. The prevailing view that intervention, and thus an appropriate workup should only be performed after failure of conservative treatment may deprive patients of early substantial symptom relief. Early investigations of postthrombotic limbs to describe the anatomic distribution of reflux and obstruction are mandatory as the conservative treatment is started. Invasive and conservative treatment may then be continued simultaneously. The decision to intervene is based upon the clinical status of the patient and by the result of adequate investigations. Minimally invasive interventions such as venous stenting and superficial reflux ablation are relatively simple with good efficacy and low risk. When obstruction is associated with reflux, the obstruction should be treated first. Concomitant superficial reflux may be treated in the same sitting, but any associated deep reflux is ignored pending clinical response to these interventions. Deep vein valve repair is considered a second-stage intervention in these limbs when conservative and minimally invasive therapy fail. Deep venous insufficiency with no outflow obstruction appears to be a major determinant for failure after control of superficial saphenous and perforator reflux in postthrombotic limbs. Therefore, it has been suggested that these procedures should be performed concomitantly with deep valve repair.
INTRODUCTION
Symptoms in patients with a previous history of deep venous thrombosis (DVT) may vary from minimal swelling to pain, skin changes, and venous ulcerations. Compression therapy is still considered by many to be the cornerstone of management of not only post-thrombotic symptoms, but of all venous disease. With a history of previous deep venous thrombosis and perceived scarred veins with ruined valves and varying degrees of obstruction, many physicians would assume that leg compression and local wound care is the only remedy. In my opinion, this is an old-fashioned and counterproductive view that may deny patients modern treatment. With new techniques available for the diagnosis and treatment of chronic venous disease, the basis for management should instead be to accurately verify and classify the presence of venous dysfunction. Treatment in symptomatic patients must take into account the degree and distribution of valve reflux and outflow obstruction. Invasive treatment should then be considered in combination with conservative measures such as compression therapy, which is certainly one aspect of the treatment.
The complexity of investigations of post-thrombotic disease depends upon the severity of symptoms and the availability of investigatory tools. Three levels have been identified by the CEAP committee of the American Venous Forum:1 Level 1: Office visit with history, clinical examination, and handheld continuous-wave Doppler; Level 2: Noninvasive vascular laboratory investigations, with mandatory duplex scanning and possibly plethysmography; Level 3: Invasive investigations or more complex imaging studies, including ascending and descending venography, varicography, venous pressure measurements, venous spiral computed tomography (CT), or magnetic resonance venography (MRV). The aim of the investigations is to describe the anatomic distribution of venous disease in the superficial, perforator, and deep systems and the presence of reflux and/or obstruction of these venous segments. In the case of significantly symptomatic post-thrombotic syndrome, level 2 and 3 investigations are usually necessary. It is possible to direct the invasive treatment correctly only with adequate knowledge of the pathophysiologic condition. The final decision to operate is based upon the clinical status of the patient rather than the test data, since the patient’s symptoms and signs may not correlate with the laboratory findings.2
Conservative or invasive treatment does not necessarily correct the basic cause of the chronic venous disease. Post-thrombotic venous disease appears to be chronic in nature. A progressive functional deterioration is observed long after the initial acute thrombosis, perhaps a result of prolonged inflammatory response, underlying thrombophilia, and subclinical recurrent events of thrombosis. Philosophically, our conceptual approach to invasive treatment of venous disease should perhaps be similar to that of arterial surgery. Like arterial bypass operations, venous surgery ameliorates the symptoms but does not cure the disease. Occlusion of a bypass inserted to treat critical ischemia does not necessarily lead to recurrence of gangrene and loss of the limb. Similarly, it has been observed that late failure of a repaired valve station with reflux does not mandate recurrence of a venous ulcer. Contrarily, recurrence of symptoms of venous and arterial disease, eg, ulcer, is not necessarily failure of treatment, but may instead represent progression of the disease. The aim of invasive treatment is to achieve a compensated state of venous function by correcting one or several factors contributing to the pathophysiology, recognizing that a completely normalized function may be impossible to achieve. For example, it has been shown that a decreased ulcer recurrence rate has been observed in limbs with less reflux as measured by air plethysmography (limbs with venous filling index (VFI) < 4.0 mL/s versus those with > 4.0 mL/s; 28% and 53%, respectively).3 Similarly it has been reported that the recurrence rate was only 14% if a venous filling time (VFT) of greater than 5s could be maintained as compared with 45% when VFT was less than 5s.4 The patient appears to show improvement even when the invasive treatments result in only partial correction of the reflux.
Groups of patients have been followed after correction of underlying venous pathology by superficial and deep venous interventions, and most investigators have found a long-term symptomatic improvement in post-thrombotic limbs. Only a few prospective studies have been reported, but they are consistent in reporting, for example, that the ulcer healing rate is shortened and ulcer recurrence rate decreased when intervention is combined with compression therapy and local ulcer treatment in limbs with combined deep and superficial disease.sup>5 It would appear logical to start with less invasive treatment initially, ie, percutaneous control of great saphenous vein (GSV) reflux or ilio-caval stenting and when minimally invasive therapy fails to proceed to open surgery, ie, valve repair or bypass surgery.
INVASIVE TREATMENT OF VENOUS OBSTRUCTION
Venous outflow obstructions are often observed following acute deep vein thrombosis due to subsequent absent or poor venous recanalization. It is found in combination with reflux in 55% of symptomatic patients, and this combination leads to the higher levels of venous ambulatory pressure and more severe symptoms then when either condition is present alone. The remaining obstruction is the principal cause of symptoms in approximately one third of post-thrombotic limbs.6,7 It appears that proximal obstruction of the venous outflow, especially the iliac vein, is more symptomatic than is segmental blockage.8,9 The collateral formation is relatively poor around an iliofemoral obstruction, contrary to the situation when the femoral-popliteal vein is blocked. Following iliofemoral DVT, only 20% to 30% of iliac veins completely recanalize spontaneously, while the remaining veins recanalize partly and develop varying degrees of collaterals.10,11 The main purpose of intervention is to relieve proximal obstruction. Most authors agree that when significant obstruction is localized above the inguinal ligament, the obstruction should be treated before any concomitant reflux. The key for the physician is to be aware of the importance and possibility of venous blockage.
Unfortunately, there are no reliable tests to measure a hemodynamically significant stenosis. Although a positive noninvasive or invasive test for obstruction may indicate the need to proceed with further investigations, a negative test should not discourage additional testing. The diagnosis of outflow obstruction is morphologic, and must be made by investigations such as ascending or antegrade transfemoral venography; intravascular ultrasound, which is superior;12,13 MRV or CT phlebography.14,15
FEMORO-ILIO-CAVAL STENTING
The introduction of percutaneous iliac venous balloon dilation and stenting has dramatically changed the treatment of the iliofemoral outflow obstruction. The endovascular technique has emerged as the efficient “method of choice” to relieve at least proximal iliofemoral obstruction. It can be offered to a larger group of patients because it is a safe and relatively simple intervention. Ultrasound-guided percutaneous cannulation is performed distal to the obstruction in the thigh portion of the femoral vein or through the popliteal vein. Partial obstruction of the post-thrombotic iliofemoral vein is usually fairly simple to transverse and treat, but even post-thrombotic limbs with occlusion can more often than not be recanalized and stented
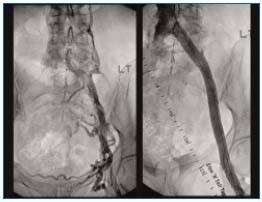
Figure 1. (Left) Left iliofemoral post-thrombotic obstruction
with axial and transpelvic collaterals. (Right) Venogram after
recanalization and stenting shows no outflow obstruction
and disappearance of collaterals.
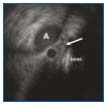
Figure 2. Intravascular ultrasound (IVUS) of the iliac vessel crossing after recanalization but before stenting. The left occluded vein (arrow) is compressed by the right common iliac artery (A) against the bone. The black circle within the vein is the IVUS catheter.
The results following venous stenting are usually poorly presented. Most studies are case reports and few have a significant number of patients; the follow-up is shortterm; patency is not reported in cumulative fashion; stented sites in the upper and lower extremities are mixed; there is no differentiation between etiologies and no separation of acute and chronic conditions. Patency rates assessed by duplex ultrasound or venography in successfully stented limbs of mixed groups of patients vary greatly. Primary and secondary patency rates 12 to 52 months after stenting are 50% to 100%, and 75% to 100%, respectively.16-19
Patency rates and in-stent recurrence of stenosis appear poorer in stented limbs with post-thrombotic disease as compared with nonthrombotic limbs. Our own experience of iliofemoral stenting has shown cumulative primary, assisted-primary and secondary patency rates at 3 years to be 75%, 92%, and 93%, respectively.20,21 The stented limbs with thrombotic disease appeared to fare significantly worse than did those with nonthrombotic disease (primary, assisted-primary, and secondary cumulative patency rates of 65%, 85% and 88%, and 89%, 100%, and 100%, respectively, at 36 months). Severe instent recurrent stenosis (ISR), ie, >50% diameter decrease on single-plane anterior-posterior venogram, is infrequent overall (only present in 15% present at 42 months).22 However, cumulative higher rates of severe ISR occurred with treatment of thrombotic as compared to nonthrombotic limbs (23% and 4%, respectively); in the presence of thrombophilia (18% and 12%, respectively); long stented area (13 to 35 cm; 25%) at 36 months. These results may reflect treatment of a more severe and extensive disease seen in limbs with postthrombotic disease. The above major risk factors for development of ISR are similar to those observed in limbs with late occlusion, although late occlusion is not necessarily preceded by increased in-stent restenosis. Other factors, such as acute recurrent thrombosis with direct occlusion of the stent or deterioration of the venous inflow, may play a major role.
The reports describing patency rates indicate clinical improvement in most patients (>72%).17,19,23 The incidence of ulcer healing after iliac vein balloon dilation and stent placement in 41 limbs with active ulcer was 68%, and the cumulative ulcer recurrence-free rate at 2 years was 62%.20 Median swelling and pain severity scores decreased significantly. The frequency of limbs with no swelling increased significantly from 12% to 47%, and limbs with no pain rose from 7% to 71%. The improvement in pain and swelling was significant in both ulcerated and nonulcerated limbs, indicating that the ulcer was not the only cause of pain and swelling. Using a quality-of-life questionnaire, the patients indicated significant improvement in all major categories after venous stenting. The results clearly indicate significant symptom relief in the intermediate term after balloon angioplasty and stent placement in the treatment of iliac venous outflow obstruction.
OPEN BYPASS SURGERY
A crossover bypass can be constructed by using either the contralateral saphenous vein (either by rotation or as a free saphenous graft) or a prosthetic graft. The autogenous cross-femoral venous bypass appears to be less thrombogenetic with better patency than prosthetic grafts, but may afford poor symptom relief owing to its small crosscut area and relatively large resistance to flow.24 This is why the size of a 10-mm ringed PTFE (PolyTetraFluoroEthylene) graft is generally recommended for bypass as an alternative to the absent or an inadequately sized saphenous vein (< 4 mm).25,26 The crossover reconstruction has been reported to be durable with good symptom relief with so-called “clinical” and venographic patency ranging from 44% to 100% with a follow-up of 5 years.26-28
The anatomic in-line bypass reconstruction can be used in the femoro-ilio-caval axial outflow axis with segmental obstruction in the presence of a sufficient venous inand outflow of the graft. Patency rates during follow-up from 1 to 150 months range from 29% to 100%.24,29 Saphenopopliteal vein bypass of femoropopliteal obstruction is rarely performed, since it requires a patent, nonvaricose great saphenous vein with competent valves and a patent tibial inflow tract. The clinical success and patency rates are poor.30,31
The results following open reconstructions have similar shortcomings as for stenting. Most vascular surgeons report a poor experience with open bypasses, with frequent early occlusion despite use of an adjunctive arteriovenous fistulae and meticulous perioperative anticoagulation. The poor patency rate is probably due to low velocity flow of the graft, external compression of the low pressure bypass, inherent thrombogenicity of the nonsaphenous graft material, and poor distal venous inflow due to extensive distal disease. Open bypass surgery, owing to its invasiveness, risky continuous anticoagulation and uncertain long-term result, should, therefore, be reserved to treat limbs after unsuccessful stenting attempts; later stent failure, which can not be adequately disobliterated; and perhaps long total occlusions, which appear to have a poorer result.
CORRECTION OF REFLUX
The only objective means of measuring advanced postthrombotic disease is to estimate the ulcer healing rate and ulcer recurrence rate in the presence of ulcer. It is much more difficult to objectively assess ulcer-free limbs for improvement in swelling, discoloration, pain, and discomfort. Most studies evaluating interventions to correct superficial and deep reflux have therefore been performed in limbs with ulcers. There are many population studies, but few appropriate prospective studies are available to assess the beneficial effect of correction of reflux on leg ulcers.
Interventions and conservative therapy should be instituted simultaneously. In patients with combined superficial and deep venous insufficiency, superficial venous surgery without compression bandaging failed to improve venous hemodynamics and achieve ulcer healing.32 On the other hand, in a prospective, non-randomized study, McDaniel et al showed a significantly smaller cumulative recurrence rate at 48 months in limbs treat ed with a variety of operations vs those treated without surgery (26% and 52%, respectively).3 They found that patients who were not candidates for surgery or who elected to forego surgery had a 3.4 times higher rate of ulcer recurrence. Another prospective, randomized study allocated ulcer limbs with isolated venous superficial incompetence or mixed superficial and deep venous reflux to either a multilayer compression treatment or a combination of compression and superficial ablative surgery. The overall 24-week healing rates were similar in the two groups, but 12-month ulcer recurrence rates were significantly reduced in the compression and surgery group as compared with the compression alone group (12% and 28%, respectively).5
The superficial ablative surgery does not improve an axial (femoropopliteal) deep venous reflux in a postthrombotic limb. Limbs with ulcer may have axial superficial reflux associated with limited segmental deep reflux. Superficial venous surgery has been shown to abolish deep venous reflux in 50% of these limbs and a 77% ulcer healing rate can be achieved at 12 months.33 It has also been feared that ablation of the superficial reflux in post-thrombotic limbs would result in worsening of the outflow obstruction by removing potential collateral circulation. Adequate deep axial collaterals, however, are invariably present in the presence of infrainguinal axial venous obstruction, even when not visualized on ascending venography. Superficial ablative surgery can be safely performed in post-thrombotic limbs.34
There is increasing support for the beneficial effect of superficial vein surgery on the healing rate and recurrence of venous leg ulcer. The ulcer recurrence rate is, however, markedly increased by the presence of deep reflux even after superficial reflux ablation.35,36 A cumulative recurrence rate at 4 to 5 years is reported to be 67% to 100%, and 6% to 28% in limbs with and without deep involvement, respectively.3,35,36 Thus, deep venous insufficiency appears to be a major determinant for ulcer recurrence. Concomitant deep and superficial repair therefore appears logical in limbs with combined deep and superficial axial reflux as an alternative to staged procedures, although this approach has not been assessed prospectively.
ABLATION OF SUPERFICIAL REFLUX
There are several methods for the treatment of truncal and nontruncal superficial reflux. It is generally accepted that liquid compression sclerotherapy is effective in the treatment of venectasias and nontruncal varicosities in the absence of GSV or short saphenous vein (SSV) trunk reflux.37 In post-thrombotic legs, sclerotherapy is frequently combined with other interventions. Sclerotherapy with foam has been shown to be superior to liquid sclerotherapy in GSV in terms of clinical and hemodynamic outcome.38,39 Treatment of limbs in clinical class C4-6 is apparently particularly rewarding.40
Saphenous vein stripping is still the standard in controlling saphenous trunk reflux. It may be combined with miniphlebectomy. Alternative catheter-based methods of endoluminal obliteration of the GSV have been developed using bipolar energy by radio frequency or laser. Both methods achieve obliteration of the GSV in 85% to 90% after 3 to 4 years.41,42 Unfortunately, the patency and competency rates were not analyzed cumulatively (Kaplan- Meier method) and are therefore of lesser value because of the substantial dropout of patients in these studies.
Only two randomized controlled trials have been reported, both comparing endovenous GSV ablation by radiofrequency (RF) to open GSV stripping in patients with varicose veins. These studies showed that postoperative pain was reduced; sick leave was shorter; and faster return to normal activities and to work was observed in the RF-treated group, but at 4 months was found to be no different from the conventionally treated group43,44 A 2-year follow-up has just been published.45 Owing to the limited number of limbs studied differences do not reach statistical significance (possible type II error). Quality of life score improves to positive values between 1 and 3 weeks after surgery in the stripping group of limbs, but it never reaches the levels observed in the group treated by RF ablation, not even after 2 years. Further randomized long-term studies of more power are currently in progress, and are necessary before endoluminal obliteration can be considered the new standard. Its specific role in patients with post-thrombotic disease has not been assessed. We have successfully combined endovenous stenting with endoluminal obliteration in patients with C4-6 and found that this is a safe and effective one-stage procedure, which is truly minimally invasive. Clinical outcome is apparently no different when radiofrequency or laser is used than in open surgery, but less bleeding results secondary to heparinization during the stenting part of the intervention.
CONTROL OF INCOMPETENT PERFORATORS
The importance of the perforator reflux in the postthrombotic limb is still debated. Perforating veins can become incompetent as a result of superficial and/or deep venous reflux, but are rarely found in isolation.46
The prevalence of IPV, as well as their diameter, volume, and velocity flow, increases linearly with clinical severity of CVI, whether or not there is coexisting deep venous incompetence.47,48 The importance in the pathophysiology of PTS remains unclear. Opinions among surgeons vary greatly, from totally ignoring incompetent perforators to detailed mapping and specific treatment. In addition, the issue is muddled by the fact that complete eradication of superficial venous reflux will lead most IPVs to be interrupted or regain competence.sup>49,50
The incompetent perforators may be controlled specifically by ultrasound-guided sclerotherapy, although longterm results do not exist. Separate multiple oblique incisions over large insufficient perforators are still used. The old Linton operations with large incisions and high wound complication rates have been abandoned for the use of subfascial endoscopic perforator surgery (SEPS). Numerous uncontrolled studies have suggested that SEPS might improve the symptoms of chronic venous disease. Unfortunately, concomitant saphenous surgery was frequently undertaken, rendering it difficult to assess whether the beneficial effect resulted from the SEPS procedure or, more likely, from the saphenous ablation.51,52 The benefits of SEPS treatment of post-thrombotic syndrome remain especially doubtful. It appears that deep venous reflux (especially if post-thrombotic) might diminish the benefits of SEPS.52,53 In such patients, the uncontrolled NASEPS registry showed that ulcer healing and ulcer recurrence rates were similar to those expected from compression therapy alone.35 Until proper prospective randomized studies have been performed and perhaps appropriate subgroups to be treated have been identified, the role of the SEPS procedure in the treatment of post-thrombotic disease remains undefined. The procedure will continue to be used by many investigators, who feel SEPS benefits the patients with postthrombotic limbs.
DEEP VEIN VALVE REPAIR
An estimated 60% to 85% of patients with deep venous reflux have had a previous deep vein thrombosis. Most commonly the valve station, which is involved in the inflammatory response elicited by the blood clot, is destroyed and cannot be repaired. In a few instances, the valve is minimally affected and can still be directly repaired. Valves above the proximal extent of the deep venous thrombus may be unaffected by the inflammatory process but are still incompetent. In fact, the first open valvuloplasty, performed by Dr Kistner, was performed in a patient with a previous distal DVT.54 An intact incompetent valve can be repaired by internal valvuloplasty, wrapping,55 and external transmural56 or transcommissural57 valvuloplasty (Figure 3). When the valve is completely destroyed the axial reflux can only be controlled by transposition58 or most commonly axillary vein autotransplantation (Figure 4).59 Other procedures such as silastic gracilis sling procedure, neovalve creation, and insertion of cryopreserved allografts, while initially promising, have now been largely discarded. Much hope is placed in percutaneously placed devices, but these are still experimental and unproven.
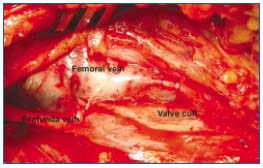
Figure 3. Transcommissural valvuloplasty. The stitches have been placed to narrow the angle between the cuff insertion lines and
tightened the valve cuffs on one side. Similar stitching is then
performed on the contralateral side.
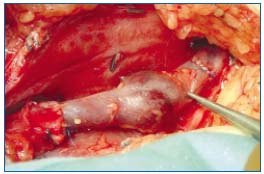
Figure 4. Axillary vein transfer to proximal femoral vein sutured
in place. Note the thinner vein wall and the well outlined cuff
insertion lines of the transplant.
Although deep valve repair appears to be beneficial in single-center studies, the proof is circumstantial, since no prospective randomized studies exist. It is unlikely that such a study will ever be performed. Deep valve reconstruction with appropriate long-term follow-up by Masuda and Kistner resulted in a 40% ulcer recurrence over a long period and many patients had long ulcer-free periods (5 to 10 years).58 Results after repair of valves in primary disease has been reported to be superior to postthrombotic disease.58,60 Raju et al reported a 6-year cumulative ulcer recurrence rate of approximately 40%, similar in primary and secondary disease after deep reconstruction.61 The result appears more related to the type of procedure (direct repair versus axillary vein transfer) than to the presence of previous thrombosis. Overall deep venous valve repair in post-thrombotic limbs has a 50% to 60% ulcer recurrence-free rate up to 10 years after the intervention.
PRACTICAL IMPLICATIONS
The treatment of a symptomatic post-thrombotic syndrome is a difficult, dynamic, and lifelong undertaking. Disabling pain and swelling are important symptoms, in addition to leg ulcers. Despite the paucity of prospective, randomized information on efficacy, it may be that open or percutaneous correction of underlying disease is currently underused. The prevailing view that intervention can only be performed after failure of conservative treatment may deprive patients of early substantial symptom relief. Invasive and conservative treatment should be used simultaneously as appropriate, and do not conflict with, but rather are complementary of, each other. The decision to intervene is based upon the clinical status of the patient; the type of intervention is directed by the result of adequate investigations. At least minimally invasive interventions such as venous stenting and superficial reflux ablation may be performed at an early stage. Significant iliofemoral venous obstruction should be treated, whether associated with reflux or not. When obstruction is shown with reflux, the obstruction should be treated first. When concomitant superficial reflux, usually GSV reflux, is present we are now increasingly combining the stenting with percutaneous catheter obliteration with or without miniphlebectomy in the same sitting. Any associated deep reflux is ignored pending clinical response to this intervention. Valve repair of associated deep reflux is considered a second-stage intervention in patients who fail conservative and minimally invasive therapy. Contrarily, as discussed above, deep venous insufficiency appears to be a major determinant for failure after ablation of superficial saphenous and perforator reflux in post-thrombotic limbs. Therefore, it has been suggested that these procedures should be performed concomitantly with deep valve repair.

REFERENCES
2 Iafrati M, O’Donnell TF. Surgical reconstruction for deep venous insufficiency. J Mal Vasc. 1997;22:193-197.
3 McDaniel HB, Marston WA, Farber MA, et al. Recurrence of chronic venous ulcers on the basis of clinical, etiologic, anatomic, and pathophysiologic criteria and air plethysmography. J Vasc Surg. 2002;35:723-728.
4 Raju S, Neglén P, Doolittle J, Meydrech EF. Axillary vein transfer in trabeculated postthrombotic veins. J Vasc Surg. 1999;29:1050-1062 (discussion 1062- 1064).
5 Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomized controlled trial. Lancet. 2004;363:1854- 1859.
6 Johnson BF, Manzo RA, Bergelin RO, Strandness DE Jr. The site of residual abnormalities in the leg veins in long-term follow-up after deep vein thrombosis and their relationship to the development of the post-thrombotic syndrome. Int Angiol. 1996;15:14-19.
7 Johnson BF, Manzo RA, Bergelin RO, Strandness DE Jr. Relationship between changes in the deep venous system and the development of the postthrombotic syndrome after an acute episode of lower limb deep vein thrombosis: a one- to six-year follow-up. J Vasc Surg. 1995;21:307-312 (discussion 313).
8 May R. Anatomy. In: Surgery of the Veins of the Leg and Pelvis. Stuttgart, Germany: Georg Thieme Verlag; 1979:1-36.
9 Mavor GE, Galloway JM. Collaterals of the deep venous circulation of the lower limb. Surg Gynecol Obstet. 1967;125:561-571.
10 Plate G, Akesson H, Einarsson E, et al. Long-term results of venous thrombectomy combined with a temporary arterio-venous fistula. Eur J Vasc Surg. 1990;4:483-489.
11 Mavor GE, Galloway JM. Iliofemoral venous thrombosis. Pathological considerations and surgical management. Br J Surg. 1969;56:45-59.
12 Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002;35:694-700.
13 Forauer AR, Gemmete JJ, Dasika NL, et al. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13:523-527.
14 Chung JW, Yoon CJ, Jung SI, et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15:249-256.
15 Fraser DG, Moody AR, Martel A, Morgan PS. Re-evaluation of iliac compression syndrome using magnetic resonance imaging in patients with acute deep venous thromboses. J Vasc Surg. 2004;40:604-611.
16 Nazarian GK, Austin WR, Wegryn SA, et al. Venous recanalization by metallic stents after failure of balloon angioplasty or surgery: four-year experience. Cardiovasc Intervent Radiol. 1996;19:227-233.
17 Hurst DR, Forauer AR, Bloom JR, et al. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34:106-113.
18 Nazarian GK, Bjarnason H, Dietz CA Jr, et al. Iliofemoral venous stenoses: effectiveness of treatment with metallic endovascular stents. Radiology. 1996;200:193-199.
19 Binkert CA, Schoch E, Stuckmann G, et al. Treatment of pelvic venous spur (May- Thurner syndrome) with self-expanding metallic endoprostheses. Cardiovasc Intervent Radiol. 1998;21:22-26.
20 Raju S, Owen S Jr, Neglén P. The clinical impact of iliac venous stents in the management of chronic venous insufficiency. J Vasc Surg. 2002;35:8-15.
21 Neglén P. Endovascular treatment of chronic iliofemoral venous obstruction – A review. Phlebolymphology. 2003;43:204- 211.
22 Neglén P, Raju S. In-stent recurrent stenosis in stents placed in the lower extremity venous outflow tract. J Vasc Surg. 2004;39:181-187.
23 O’Sullivan GJ, Semba CP, Bittner CA, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11:823-836.
24 Jost CJ, Gloviczki P, Cherry KJ Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320-327 (discussion 327-328).
25 Lalka SG, Lash JM, Unthank JL, et al. Inadequacy of saphenous vein grafts for cross-femoral venous bypass. J Vasc Surg. 1991;13:622-630.
26 Eklof B, Albrechtson U, Einarsson E, Plate G. The temporary arteriovenous fistula in venous reconstructive surgery. Int Angiol. 1985;4:455-462.
27 Halliday P, Harris J, May J. Femorofemoral crossover grafts (Palma operation): a long-term follow-up study. In: Surgery of the Veins. Orlando, Fl: Grune & Stratton; 1985:241-254.
28 Hutschenreiter S, Vollmar J, Loeprecht H, Abendschein A, Rodl W. Rekonstruktive Eingriffe am Venensystem: Spatergebnisse unter Kritischer Bewertung funktioneller und gefassmorphologischer Kriterien. Chirurg. 1979;50:555-563.
29 Alimi YS, DiMauro P, Fabre D, Juhan C. Iliac vein reconstructions to treat acute and chronic venous occlusive disease. J Vasc Surg. 1997;25:673-681.
30 AbuRahma AF, Robinson PA, Boland JP. Clinical, hemodynamic, and anatomic predictors of long-term outcome of lower extremity venovenous bypasses. J Vasc Surg. 1991;14:635-44.
31 Husni EA. Clinical experience with femoropopliteal venous reconstruction. In: Venous Problems. Chicago, Il: Yearbook Medical Publishers; 1978:485-491.
32 Scriven JM, Hartshorne T, Thrush AJ, et al. Role of saphenous vein surgery in the treatment of venous ulceration. Br J Surg. 1998;85:781-784.
33 Adam DJ, Bello M, Hartshorne T, London NJ. Role of superficial venous surgery in patients with combined superficial and segmental deep venous reflux. Eur J Vasc Endovasc Surg. 2003;25:469-472.
34 Raju S, Easterwood L, Fountain T, Fredericks RK, Neglén PN, Devidas M. Saphenectomy in the presence of chronic venous obstruction. Surgery. 1998;123:637-644.
35 Gloviczki P, Bergan JJ, Rhodes JM, et al. Mid-term results of endoscopic perforator vein interruption for chronic venous insufficiency: lessons learned from the North American subfascial endoscopic perforator surgery registry. The North American Study Group. J Vasc Surg. 1999;29:489-502.
36 Burnand K, Thomas ML, O’Donnell T, Browse NL. Relation between postphlebitic changes in the deep veins and results of surgical treatment of venous ulcers. Lancet. 1976;1:936-938.
37 Rigby KA, Palfreyman SJ, Beverley C, Michaels JA. Surgery versus sclerotherapy for the treatment of varicose veins. Cochrane Database Syst Rev. 2004:CD004980.
38 Hamel-Desnos C, Desnos P, Wollmann JC, et al. Evaluation of the efficacy of polidocanol in the form of foam compared with liquid form in sclerotherapy of the greater saphenous vein: initial results. Dermatol Surg. 2003;29:1170-1175 (discussion 1175).
39 Yamaki T, Nozaki M, Iwasaka S. Comparative study of duplex-guided foam sclerotherapy and duplex-guided liquid sclerotherapy for the treatment of superficial venous insufficiency. Dermatol Surg. 2004;30:718-722 (discussion 722).
40 Cabrera J, Redondo P, Becerra A, et al. Ultrasound-guided injection of polidocanol microfoam in the management of venous leg ulcers. Arch Dermatol. 2004;140: 667-673.
41 Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
42 Merchant RF, Pichot O, Myers KA. Four-year follow-up on endovascular radiofrequency obliteration of great saphenous reflux. Dermatol Surg. 2005;31:129-134.
43 Rautio T, Ohinmaa A, Perala J, et al. Endovenous obliteration versus conventional stripping operation in the treatment of primary varicose veins: a randomized controlled trial with comparison of the costs. J Vasc Surg. 2002;35:958-965.
44 Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003;38:207-214.
45 Lurie F, Creton D, Eklof B, et al. Prospective randomised study of endovenous radiofrequency obliteration (closure) versus ligation and vein stripping (EVOLVeS): two-year follow-up. Eur J Vasc Endovasc Surg. 2005;29:67-73.
46 Myers KA, Ziegenbein RW, Zeng GH, Matthews PG. Duplex ultrasonography scanning for chronic venous disease: patterns of venous reflux. J Vasc Surg. 1995;21:605-612.
47 Stuart WP, Adam DJ, Allan PL, et al. The relationship between the number, competence, and diameter of medial calf perforating veins and the clinical status in healthy subjects and patients with lowerlimb venous disease. J Vasc Surg. 2000;32:138-143.
48 Delis KT, Husmann M, Kalodiki E, et al. In situ hemodynamics of perforating veins in chronic venous insufficiency. J Vasc Surg. 2001;33:773-782.
49 Stuart WP, Adam DJ, Allan PL, et al. Saphenous surgery does not correct perforator incompetence in the presence of deep venous reflux. J Vasc Surg. 1998;28:834-838.
50 Al-Mulhim AS, El-Hoseiny H, Al-Mulhim FM, et al. Surgical correction of main stem reflux in the superficial venous system: does it improve the blood flow of incompetent perforating veins? World J Surg. 2003;27:793-796.
51 Jeanneret C, Fischer R, Chandler JG, et al. Great saphenous vein stripping with liberal use of subfascial endoscopic perforator vein surgery (SEPS). Ann Vasc Surg. 2003;17:539-549.
52 Bianchi C, Ballard JL, Abou-Zamzam AM, Teruya TH. Subfascial endoscopic perforator vein surgery combined with saphenous vein ablation: results and critical analysis. J Vasc Surg. 2003;38:67-71.
53 Kalra M, Gloviczki P. Subfascial endoscopic perforator vein surgery: who benefits? Semin Vasc Surg. 2002;15:39-49.
54 Kistner RL. Surgical repair of the incompetent femoral vein valve. Arch Surg. 1975;110:1336-1342.
55 Lane RJ, Cuzzilla ML, McMahon CG. Intermediate to long-term results of repairing incompetent multiple deep venous valves using external valvular stenting. ANZ J Surg. 2003;73:267-274.
56 Kistner RL. Surgical technique: External venous valve repair. Straub Found Proc. 1990;55:15-16.
57 Raju S, Berry MA, Neglén P. Transcommissural valvuloplasty: technique and results. J Vasc Surg. 2000;32:969-976.
58 Masuda EM, Kistner RL. Long-term results of venous valve reconstruction: a four- to twenty-one-year follow-up. J Vasc Surg. 1994;19:391-403.
59 Taheri SA. Venous reconstruction in venous insufficiency syndrome. Del Med J. 1988;60:425-427.
60 Perrin M. Reconstructive surgery for deep venous reflux: a report on 144 cases. Cardiovasc Surg. 2000;8:246-255.
61 Raju S, Fredericks RK, Neglén PN, Bass JD. Durability of venous valve reconstruction techniques for “primary” and postthrombotic reflux. J Vasc Surg. 1996;23:357-366 (discussion 366-367).
