Interaction between ageing, inflammation process, and the occurence of varicose veins
Gemma Pascual
Juan M. Bellón
Department of Surgery
Faculty of Medicine, University of Alcalá
Alcalá de Henares
Madrid, Spain

SUMMARY
Failure of the vein wall continues to be a subject of intense research, the aim of which is to establish a cause that will allow us to prevent or at least slow down this undesirable process. We have investigated the role played by inflammatory cells, especially leukocytes, in the etiology of varicose veins. We hypothesize that venous insufficiency could result from the interaction between two processes: tissue aging and abnormalities in the leukocyte/endothelial cell interaction. These two processes, the individual contribution of which depends on the context, are believed to compound each other resulting in venous insufficiency. Thus, in young individuals, venous insufficiency might be induced by accelerated tissue aging, whereas in older subjects, it could be due to an interaction between the aging process, which is slower and prolonged, and abnormalities in the interaction between
leukocytes and endothelial cells.
The venous circulation is today perhaps among the most well-understood systems of the human body. However, the insidious and often prolonged nature of venous insufficiency hinders investigation into the factors that could trigger this disease, the progression of which is intimately linked to the natural process of tissue aging. Hence, in this area of medicine, as in many others grouped under the term “chronic,” the clear conscience of the clinician occurs at the expense of the despair of the specialist. We believe that through analyzing some aspects of our understanding of this “silent” disease, patients, doctors, and health care managers will soon act together in combating venous disease.
Chronic venous insufficiency (CVI) as a clinical definition indicates a loss in efficiency of normal vein function. Its symptoms, however, are a product of effects secondary to the inefficiency of the system, and thus the origin of this disease is not yet clearly established. The vein wall is a highly dynamic structure with 2 welldifferentiated sides that guarantee its correct functioning: the luminal and adventitial sides. The luminal side of the vein wall, where the presence of valves indicates that the complex interaction between blood and wall takes on a particularly dynamic and metabolic dimension, is lined with an endothelium whose characteristics differ from the well-known properties of the arterial or microvascular endothelium. The adventitial side, the site of mechanical support and nutrient intake for the entire wall, also plays an active role in fighting against the forces of gravity. This complex role of the vein wall is, nevertheless, discrete and any decrease in its efficiency runs a slow course; hence its ambiguous assigning to the group of “chronic diseases.”
Our interest lies in the role that certain endogenous or environmental factors have in the development of CVI. Among the intrinsic factors, aging is being ascribed an ever more relevant role. Some of the genetic features of the aging process, such as the loss of function of telomerases and their involvement in maintaining the cell population or changes in the expression (dysregulation) of other genes, lead to qualitative and quantitative changes in cellular and extracellular proteomics that enter into the realm of consequences more than cause.
The participation of white blood cells where they release in the development of venous disease is well established,1-3 and aggregated when they are known to be the most common cause of venous stasis. Stasis of blood flow, whether at the luminal level due to valve failure or at the adventitial microvascularization level, triggers the activation of leukocytes as part of an inflammatory process aimed at functional recovery and tissue repair. Hence, what in principle is a protective mechanism can turn hostile to the detriment of the blood vessels.
Little is known about how CVI leads to the local tissue destruction seen so commonly in clinical practice.1 Increased evidence points to a central role for abnormal leukocyte–endothelial cell interactions in the pathogenesis of this condition.4-6 Some theories have suggested that leukocytes are sequestered in the legs of patients with CVI. This sequestering leads to the activation of white cells, which results in the generation of free radicals, proteases, histamine, neutrophil chemoattractants, and complement.7-10 The actions of these substances destroy the endothelial layer of the vessel and their basement membranes, increasing vascular permeability and disturbing microcirculatory flow.11,12 Other authors propose other factors that might activate leukocytes and cause them to act inappropriately in the venous system. Some factors present in the plasma of patients can activate unstimulated leukocytes.13 Such a factor could be any of a variety of stimuli, including bacteria, fungi, and their products. Endothelial cells also need to be activated so that leukocytes can migrate into the tissue through the endothelium. Scarce cytokine expression has been observed in some investigations, even though a considerable number of monocytes have been noted to adhere to the endothelium and migrate into tissue, suggesting that factors other than inflammatory mediators (high pressure, low shear stress) may activate the endothelium.14
Little is known about the role of inflammatory cells in the biochemical and histological changes observed in varicose disease. Increased numbers of macrophages/monocytes and mast cells have been observed in varicose veins,15,16 suggesting that vein damage in refluxing saphenous veins is associated with a leukocyte infiltrate.15 Mononuclear cells such as neutrophils normally circulate in a quiescent state, but when activated are capable of adhering to the endothelium and entering tissues a variety of noxious substances known for their ability to cause tissue damage.1 Active proteases can either be secreted directly by inflammatory cells, including elastase and cathepsin G produced by polymorphonuclear leukocytes, chymase and try ptase by mast cells, and granzymes by lymphocytes, or can be generated from circulating zymogens by activation in close contact with the cells.17
The aim of the present study was to establish the influence of age on the changes that occur in the vein wall and to characterize leukocyte infiltration in the wall, exploring its association with the varicose condition.
PATIENTS AND METHODS
Forty vein specimens were obtained during surgery from patients undergoing bypass surgery (controls) or surgery for varicose veins. All 40 subjects gave their informed consent to participate in this study. The vein specimens were first visually checked for the presence of damaged areas and then divided according to subject age to establish the following study groups:
Group I control (n=20)
This group comprised 10 vein specimens harvested from patients under 50 years of age (mean 38±8.8; range 36-45 years) and a further 10 specimens from patients aged 50 years or over (mean 71.5±10.6; range 57-89 years). These segments of saphenous vein were obtained from patients with no history of venous insufficiency or proven reflux during aortocoronary bypass surgery.
Group II varicose veins (n=20)
This group comprised 10 vein specimens harvested from patients under 50 years of age (mean 39.4±6.8; range 26-46 years) and a further 10 specimens from patients aged 50 years or over (mean 60.7±9.4; range 52-70 years). This time, the portions of saphenous vein were obtained during vein stripping from patients with primary venous insufficiency and clinically confirmed reflux.
Inflammatory cells
The detection and quantification of inflammatory cells was undertaken using immunohistochemical techniques. For the identification of CD4/CD8 and CD68 cells, tissue samples were fixed in 10% formaldehyde, embedded in paraffin, and cut into 5-mm slices using a microtome (Microm, Barcelona, Spain). The sections were then deparaffinated, hydrated, and equilibrated in phosphate-buffered saline (PBS) buffer (pH 7.4). Pretreatment of tissue by heat-induced epitope retrieval was required. This involved immersing the tissue in 10 mM citrate buffer pH 6.0 and microwave boiling for 2 minutes. Acetone-fixed frozen sections were used to identify CD19-positive cells and neutrophil collagenase (matrix metalloproteinase [MMP] 8).
We used as primary antibodies a mouse monoclonal anti-human CD68 (1:50) (DakoCytomation, Glostrup, Denmark) to identify macrophages/monocytes, mouse monoclonal anti-human CD4 (1:10) (Neomarkers, Fremont, Calif) and CD8 antibodies (1:50) (DakoCytomation, Glostrup, Denmark) to identify T cells, a mouse monoclonal anti-human CD19 antibody (1:100) (Neomarkers, Fremont, Calif) to identify B cells, and a mouse monoclonal anti-human MMP8 (1:200) (Chemicon, Temecula, Calif) to identify neutrophils. The antigen-antibody reaction was detected by the alkaline phosphatase- labeled avidin-biotin procedure. The chromogenic substrate contained alpha-naphthol and fast red. Nuclei were counterstained with Carazzi hematoxylin. After the immunohistochemical procedure, the tissue sections were examined under a light microscope (Zeiss, Jena, Germany). Infiltrated cells were counted under the microscope (_200) in 4 areas of 0.5 mm2 per patient (40 high-power fields per group). All values were expressed as means±SE. Data were compared using the Student t test. The level of significance was set at P<0.05.
RESULTS
Inflammatory cells
In general, we observed increased numbers of CD4+ cells, B cells (CD19+), and monocytes/macrophages (CD68+) in the varicose veins with respect to the normal patients. No differences were observed in CD8 and neutrophils compared with control veins. In addition, we examined the distribution of these cells as an indication of the inflammatory environment in aging and CVI. In the control vein specimens, CD4+ cells were scarce, appearing mainly in the adventitial layer in segments obtained from the young subjects (Figure 1a) and more toward the media and infiltrating the valves in control specimens from older subjects. Varicose veins showed significantly increased numbers of CD4+ cells compared with controls (Figure 1c) (P<0.005). In specimens from the older subject group, CD4+ became infiltrated in the subendothelium and valves (Figure 1b), while in the varicose vein/young specimens these cells were mainly observed in the adventitial and medial layers. Varicose veins showed significantly increased CD4+ cells compared with normal veins, in the younger population (Figure 1d). These results appear to indicate that the presence of CD4+ cells is related to the varicose condition.
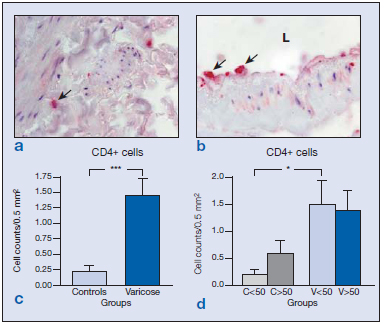
Figure 1. Immunohistochemical detection of CD4+ cells ( ) in the control veins (a) and varicose veins of an older patient (b). Quantification of the stained cells in the different groups excluding the age factor (c) showing significant differences (***P<0.005) between varicose and normal veins due to the disease process. Quantification and statistical analysis including the age of the patients (d). Note that this factor did not affect the number of CD4+ cells (*P<0.05).
Abbreviation: C, control; L, lumen; V, varicose vein.
Immunolabeling for CD8 cells was scarce both in the vein wall of healthy and varicose specimens and appeared in the adventitial and medial layers (Figure 2a and b). Differences between the groups were not found (Figure 2c and d). Only vein specimens from one subject, in which areas of hemorrhage and inflammation were observed, showed significantly higher numbers of CD8+ cells. This subject was therefore excluded from the study. B cells were identified by the CD19 antibody. The distribution of CD19+ cells was conditioned by age. In the wall of specimens from young subjects, B cells mostly appeared in the endothelium (Figure 3a), whereas in vein specimens from the older subjects (Figure 3b), CD19+ cells were mainly confined to the adventitial layer, regardless of the varicose or healthy condition. B-cell numbers were significantly higher in varicose veins compared with healthy veins (Figure 3c) (P<0.005). When stratified by age, this difference was only maintained for the group of older subjects (Figure 3d) (P<0.01).
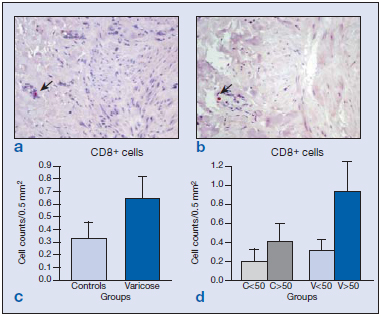
Figure 2. Image of the immunohistochemical detection of the number of CD8+ cells ( ) in the vein walls of healthy specimens (a) and varicose specimens (b). Quantification of CD8-labeled cells in the different groups excluding the age factor (c) showing no significant differences between the varicose and normal veins. Differences were also not significant when the different age-groups were compared (d).
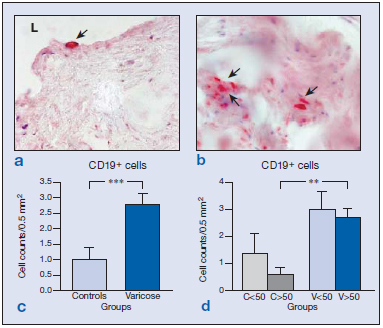
Figure 3: Immunohistochemical detection of CD19+ cells ( ) in control (a) and varicose veins (b). Quantification of positive cells in both groups excluding the age factor (c) showing significant differences (***P<0.005) attributed to the disease process. When stratified by age (d), differences between control and varicose veins were only maintained in the older study population (**P<0.01).
Abbreviations: C, control; L, lumen; V, varicose vein.
In control specimens, CD68+ cells were observed in the lower layers of the tunica media (Figure 4a), while in the varicose vein specimens, these cells infiltrated the upper areas of the media.
Higher numbers of CD68+ cells (macrophages/ monocytes) were recorded in the varicose group (Figure 4c) (*P<0.05). In specimens from young persons, the odd CD68+ cell appeared throughout the vessel wall with the exception of the intimal layer of the vein. In the specimens from elderly subjects, these cells were observed in the upper media layer, endothelium, and valves.
The most notable changes in the distribution of CD68+ cells in varicose vein specimens were the presence of monocytes/macrophages at the valves and nearby endothelium (Figure 4b). In addition, significantly higher numbers of these cells were detected in the older varicose veins (P<0.05).
When stratified by age, higher numbers of CD68+ cells were detected in both healthy and varicose specimens from the older subject group (Figure 4d). Among the specimens from older subjects, significantly more CD68+ cells were detected in the varicose veins than in the controls (P<0.01) (Figure 4d). Hence, overall there were clear differences attributable to age and the varicose condition in the distribution patterns and the numbers of CD68+ cells. These cells were also found to be associated with valve failure.
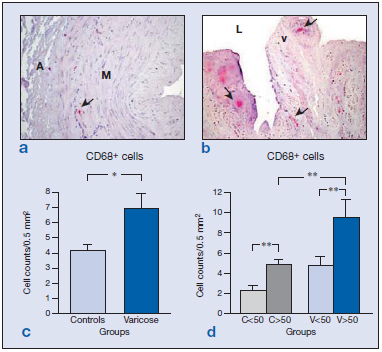
Figure 4: Images showing the immunohistochemical detection of CD68+ cells ( ) in control (a) and varicose veins (b). Regardless of age (c), the varicose state was related to a significantly increased infiltration of CD68+ cells in the vein wall (*P<0.05). Quantification and statistical analysis including the age of the patients (d) indicated that this factor significantly increased the number of labeled cells in the two groups of patients (**P<0.01). Abbreviations: A, adventitia; L, lumen; M, media; v, valve.
MMP8 is a type II collagenase secreted by neutrophils. The expression of this enzyme was not observed in the control vein specimens from the younger group (Figure 5a). In healthy specimens from the older subjects, MMP8 labeling appeared in areas of the tunica media and adventitia corresponding to degranulated neutrophils (Figure 5b). In specimens from young subjects with varicose veins, small numbers of nondegranulated cells could be seen, in all the layers of the vein wall. In the varicose vein specimens from older subjects, MMP8+ cells were found in the valves (Figure 5d), intima, adventitia (Figure 5c), and vasa vasorum of the vein wall, many of which were already degranulated. Hence, the most outstanding finding related to this enzyme was neutrophilic degranulation related to age regardless of the healthy or varicose condition of the veins.
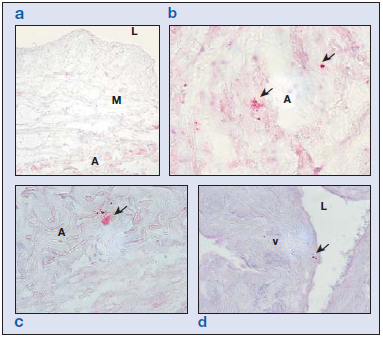
Figure 5. Immunohistochemical matrix metalloproteinase (MMP) 8 study showing staining for neutrophils ( ). In the control group of patients younger than 50 years (a), we observed no neutrophils in the vein wall. In control (b) and varicose (c) specimens from the older subjects, some preserved cells were observed in the adventitial layer and other neutrophils appeared degranulated. In the older control specimens, some neutrophils could also be observed on the valves (d).
Abbreviations: A, adventitia; L, lumen; M, media; v, valve.
DISCUSSION
In previous papers,18 aging was established as an important factor responsible for changes in the vein wall, and these changes were similar to those produced at the different stages of CVI. In this study, we tried to separate the effects of these 2 overlapping processes particularly those related to the inflammation process contributing to the general mechanism of aging (physiological) or the inflammatory response to disease (venous insufficiency). The basal state of vein wall is characterized by a special property of microcirculation venules, where the wall shear stress is low and endothelial cells can recruit inflammatory cells. In young people, white blood cells are rarely seen in the adventitial microvessels. During aging, we observed the movement of white blood cells from the adventitia to the medial layer and the upper part of the vein wall, along with the presence of white blood cells at the level of the valves, especially CD4+ and CD68+. These types of inflammatory macrophages/ monocytes play a key role in tissue remodeling due to their ability to release MMPs, growth factors, and proinflammatory cytokines. In addition, some authors advocate that macrophages/monocytes can enhance cell adhesion molecule expression by the vascular endothelium19 and induce changes in the smooth muscle phenotype.20 It would be interesting to determine whether the presence of these cells was a consequence or cause of vein wall remodeling during aging. Whichever the case, the discrete presence of these cells from the microvessels toward the vein interior would be consistent with the immunohistochemical changes in collagen and MMPs in the aging vein wall21 and with the reduction in elastin components and increased elastase activity previously described by us.22 The presence of these inflammatory cells in the medial layer of the wall could be the triggering factor for remodeling of the wall, perhaps as the consequence of a discrete but sustained chronic inflammatory process.
When we evaluated the appearance of white blood cells in varicose processes, we found a general significant increase in all the cells examined (except CD8 and neutrophils) with respect to control, healthy veins, once again supporting the participation of inflammatory cells in the changes that affect the insufficient vein wall. Nevertheless, the distribution of these cells in the vein wall and the effects of age also need to be established.
In young CVI patients, CD4+ T lymphocytes were detected mainly in the adventitial area as occurred in the control group, only these appeared in significantly greater numbers. In the vein specimens from older subjects, a change was observed in the distribution of these cells, which accumulated at the level of the valves and in adjacent endothelial and subendothelial areas. Our findings differ in part from those of other authors,16 who described no significant increase in the number of T lymphocytes in varicose veins. The increased number and more importantly the distribution of CD4+ cells confirm the changes that occur at the valve and luminal surface of the insufficient vein in older patients.
CD4+ lymphocytes, depending on the types of cytokine they produce, can be of the T helper (Th) 1 or Th2 type.23 Th1 lymphocytes secrete interleukin (IL) 2 and interferon gamma, which induce the inflammatory response through 2 mechanisms: facilitating the cell immune reaction and stimulating B cells. Th2 lymphocytes produce IL-4, IL-5, IL-9, IL-10, and IL-13. This array of cytokines is, in part, responsible for activating B cells. The increased numbers of the CD19+ population in the varicose condition could be correlated with the change from an inflammatory status provoked by the CD4+ cells. Other authors24 find no changes in B lymphocytes (CD20+,CD30+), although we observed the expression of the CD19+ epitope in all B cells except plasma cells, indicating the wide distribution range of this cell type.
It is well known that macrophages/monocytes accumulate at varicose vein valves, and they are more commonly observed adhering to the valve and to the vein wall above the valve complex,24 suggesting a role in the genesis of primary vein dysfunction.15 These findings confirm those of other studies16 in which macrophages/monocytes and mast cells were differently distributed throughout the vessel wall, showing a significant increase in the varicose vein wall, compared with controls. Takase et al14 observed CD68+ macrophages on the endothelium, subendothelium, and all other areas of the insufficient vein wall. We propose that the presence of these cells on the luminal surface and in particular on the valves is a good indication of valve dysfunction, a rationale supported by its significant increase with age. The set of varicose vein specimens from the older subjects showed a greater accumulation of macrophages both in the areas of the vein wall and along the valves. The disease thus enhances this difference.
The increase in CD68+ cells has been correlated with the overexpression of transforming growth factor β(TGF-β) and that of inducible nitric oxide synthase (iNOS)24 with the extent of damage. These findings are in agreement with previous results from our laboratory. Thus, we detected increased TGF-βlevels in the veins of CVI patients,22 which, added to the augmented CD68+ cells detected here, would support Jacob’s findings.
The deposition of these cells in the valve area in CVI allows us to establish an infiltration gradient during the aging and disease process, which is probably related to the changes in pressure and shear stress at the level of the valves proposed by Takase et al.14
Finally, we examined the part played by neutrophils in these processes. In the literature regarding the genesis of CVI, authors such as Sayer16 report no difference in the behavior of these cells in the vein wall, although others have described differences in the peripheral circulation. According to some authors, in the disease state, neutrophils in the blood are activated through the mediation of enzymes secreted upon the degranulation of neutrophils.25,26 Our findings are not completely in line with this theory concerning neutrophil activation (as measured by their degranulation), since we observed neutrophil degranulation in both control and varicose vein specimens from older subjects, suggesting an indirect measure of vein wall ischemia. This factor would support the chronicity of the process.
In summary, the findings of our study indicate a clear increase in the inflammatory environment provoked by aging, which starts with the vasa vasorum and goes on to markedly affect the valves in the vein wall. In our young patients with CVI, most damage appears in the luminal area and this damage then becomes more generalized in older patients with CVI, particularly at the level of the valves.
It may therefore be concluded that inflammatory cells play a pivotal role both in the aging process and the varicose process. The distribution of these cells is a good indicator of the state of the vein wall and also allows us to infer that dysfunction of the microvascular endothelium is the primary effect related to age, while valve dysfunction is most marked in venous insufficiency. Although the disease and aging processes run a parallel, overlapping course, the aging process may be accelerated in CVI coinciding with the remodeling of the vein wall affecting both its cellular component18,21,22 and its extracellular component, as observed in our previous work.

References
2. Michiels T, Arnould D, Janssens K, Bajou I, Remacle J. Interactions between endothelial cells and smooth muscle cells after their activation by hypoxia. Int Angiol.1996;15:124-130.
3. Coleridge Smith PD. Neutrophil activation and mediators of inflammation in chronic venous insufficiency. J Vasc Res.1999; 36 suppl 1):24-36.
4. Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration. A new hypothesis. BMJ. 1988;296:1726-1727.
5. Nash G, Shearman C. Neutrophils and peripheral arterial disease. Critical Ischaemia.1992;2:5-13.
6. Nash G, Shearman C. Neutrophils and peripheral arterial disease. Critical Ischaemia.1992;2:15-21.
7. Belch JJF, Chopra M, Hutchinson S, et al. Free radical pathology in chronic arterial disease. Free Radic Biol Med.1989;6:375-378.
8. Gill DS, Barradas MA, Fonseca VA, Gracey L, Dandona P. Increased histamine content in leukocytes and platelets with peripheral vascular disease. Am J Clin Pathol.1988;89:622-626.
9. Cambria RA, Anderson RJ, Dikdan J, Lysz TW, Hobson RW. The influence of arachidonic acid metabolites on leucocyte activation and skeletal muscle injury after ischaemia and reperfusion. J Vasc Surg.1991;14:549-556.
10. Bengston A, Holmberg P, Heidman M. The ischaemic leg as a source of complement. Br J Surg.1987;74:697-700.
11. Smedley LA, Tonneson MG, Snadhus RA, et al. Neutrophil mediated injury to endothelial cells: enhancement by endotoxin and the essential role of neutrophil elastase. J Clin Invest.1986; 77:1233-1243.
12. Weiss SJ. Tissue destruction by neutrophils. N Eng J Med. 1989;320: 365-376.
13. Takase S, Schmid-Schonbein G, Bergan JJ. Leukocyte activation in patients with venous insufficiency. J Vasc Surg. 1999;30: 148-156.
14. Takase S, Bergan JJ, Schmid-Schonbein G. Expression of adhesion molecules and cytokines on saphenous veins in chronic venous insufficiency. Ann Vasc Surg. 2000;14:427-435.
15. Ono T, Bergan JJ, Schmid-Schonbein GW, Takase S. Monocyte infiltration into venous valves. J Vasc Surg. 1998;27:158-166.
16. Sayer GL, Smith PDC. Immunocytochemical characterisation of the inflammatory cell infiltrate of varicose veins. Eur J Vasc endovasc Surg. 2004;28:479-483.
17. Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol. 2003;23:2146-2154.
18. Buján J, Jiménez-Cossio JA, Jurado F, et al. Evaluation of the smooth muscle cell component and apoptosis in the varicose vein wall. Histol Histopathol. 2000;15:745-752.
19. Chester AH, Morrison KJ, Yacoub MH. Expression of vascular adhesion molecules in saphenous vein coronary bypass grafts. Ann Thoracic Surg. 1998;65:1685-1689.
20. Campbell JH, Campbell GR. The cell biology of atherosclerosis-new developments. Aust N Z J Med. 1997;27:497-500.
21. Buján J, Jurado F, Gimeno MJ, et al. Changes in metalloproteinase (MMP-1, MMP-2) expression in the proximal region of the varicose saphenous vein wall in young subjects. Phlebology. 2000;15:64-70.
22. Buján J, Gimeno MJ, Jiménez JA, Kielty CM, Mecham RP, Bellón JM. Expression of elastic components in healthy and varicose veins. World J Surg. 2003;27:901-905.
23. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med.1989;170:2081-2095.
24. Jacob T, Hingorani A, Ascher E. Overexpression of transforming growth factor-ß1 correlates with increased synthesis of nitric oxide synthase in varicose veins. J Vasc Surg. 2005;41:523-530.
25. Shields DA, Andaz SK, Abeysinghe RD, Porter JB, Scurr JH, Coleridge Smith PD. Neutrophil activation in experimental ambulatory venous hypertension. Phlebology.1994;9:119-124
26. Shields DA, Andaz SK, Timothy-Antoine CA, Scurr JH, Porter JB. CD11b/CD18 as a marker of neutrophil adhesion in experimental ambulatory venous hypertension. Phlebology.1995;10 (suppl 1):108-109.
