Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment
A. Yu. Tsoukanov,
A. Nikolaychuk
Abstract
Aim: the study was aimed at investigating the frequently encountered clinical group of patients presenting with subjective leg symptoms without visible signs of chronic venous disorders. The great saphenous vein (GSV) of such patients was investigated using duplex scanning (DS) to verify whether a reflux could occur in certain circumstances, ie, at the end of the day in an orthostatic position. If there was a reflux, the possibility to eliminate it with a drug treatment, ie, MPFF, was assessed.
Material and methods: women consulting for complaints related to chronic venous disorders (CVDs) of the lower extremities, but without visible signs; therefore, with a C0s, En, An, Pn classification according to the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification, were enrolled. Symptoms were assessed using a 10 cm visual analogue scale (VAS) and the patients’ quality of life was assessed using the ChronIc Venous Insufficiency Quality of life questionnaire-20 items (CIVIQ-20). Patients underwent a DS of the lower extremities in the upright position twice a day: once in the morning (before 10 am) and once in the evening after normal physical efforts (after 6 pm). The investigations included the following measurements: (i) reflux duration; (ii) GSV diameter in the groin area (mm); and (iii) difference in the GSV diameter between the evening and morning values (mm). Patients with evening reflux received a 2-month pharmacological treatment with MPFF (1000 mg of MPFF once a day in the morning). A DS investigation was repeated after 2 months of treatment in these patients.
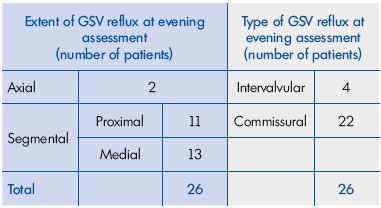
Results: a total of 41 C0s women aged 21 to 57 years (mean age, 35.4±15.1 years) were enrolled in the study and then investigated with DS. A total of 15 of the patients had no reflux, either in the morning or in the evening. The remaining 26 patients had no reflux in the morning, but presented with an evening GSV reflux. As for reflux extent, 2 had an axial reflux and 24 had a segmental reflux, of which 11 were proximal and 13 were medial. Regarding reflux type, 4 had an intervalvular reflux and 22 had a commissural reflux. The evening GSV diameter in the subgroup with reflux (n=26) was significantly larger (P<0.05), compared with patients without evening reflux (n=15; 6.33 mm [95% confidence interval (CI), 4.50- 8.00 mm] vs 5.45 mm [95% CI, 4.00-6.50 mm]). This was also true for the difference in GSV diameter between the evening and morning (0.82 mm [95% CI, 0.30-1.20 mm] vs 0.42 mm [95% CI, 0.10-0.65 mm]). Of the 26 patients with previous baseline evening GSV reflux who were investigated after a 2-month MPFF treatment, 22 no longer had reflux at 6pm, and 4 had a nonsignificantly reduced length of reflux. In parallel, the GSV diameter decreased from 6.33 mm (95% CI, 4.50-8.00 mm) to 5.50 mm (95% CI, 1.10- 7.00 mm), and the difference in GSV diameter between the evening and morning decreased from 0.82 mm (95% CI, 0.30-1.20 mm) to 0.37 mm (95% CI, 0.10-0.70 mm; P=0.000008). There was a parallel significant decrease in the intensity of subjective symptoms as demonstrated by the VAS score and a significant improvement in the patients’ quality of life after treatment (P=0.00001 for both).
Conclusion. C0s, En, An, Pn patients may present with a transient reflux in the GSV that occurs at the end of day. Objective abnormalities can be detected by DS investigations and by measuring the difference in GSV diameter between the morning and the evening. Treatment with MPFF (1000 mg of MPFF once a day in the morning for 2 months) resulted in the elimination of the evening GSV reflux in most of the treated patients, decrease in vein diameter and also resulted in beneficial effects on symptom relief and quality of life improvement.
Introduction
The C0s, En, An, Pn patient according to the Clinical, Etiological, Anatomical, Pathophysiological (CEAP) classification describes a particular kind of patient with chronic venous disorder-related symptoms, such as heavy legs in the upright position, restless legs, sensation of swelling particularly in the evening, but without visible signs of chronic venous disorders (CVDs).1,2
Despite the fact that the condition is often encountered in daily practice, the so-called phlebopathic symptoms are scarcely broached in the literature. Although, it is hard to interpret the meaning of these symptoms when there are no objective signs of CVDs.
In the 80s’, the Italian group of Andreozzi et al indicated that the prevalence of such subjects was about 20% of all people visiting their vascular lab with a suspicion of CVDs.3 Thirty years later, the same prevalence of C0s subjects was found in the Vein Consult Program, which included more than 90 000 subjects consulting their practitioner in 23 countries.2 However, the Italian team was able to find some objective abnormalities in the venous tone by plethysmographic investigations and by measuring the difference between the venous diameters in the supine and in the upright position using duplex scanning (DS). At that time, associated symptoms, such as leg pain and heaviness, were attributed to an increase in venous wall compliance. Therefore, the “Pn” scoring for “no pathophysiology detectable” might be disputed.
Since then, theories have evolved and it seems important to remember that venous valve incompetence is central to the venous hypertension that appears to underlie most or all of the symptoms and signs typically associated with CVDs.4 In most cases, venous hypertension is caused by reflux through incompetent valves in the superficial venous system. Reflux and subsequent hypertension promote chronic venous inflammation, which is likely to be responsible for the disease progression toward complications. Genetic risk factors, hormonal impregnation, prolonged hydrostatic load, and abnormal fluid shear stress may serve as mechanisms that lead to a cascade associated with an aseptic inflammation.5 Activated endothelium, leukocytes, mast cells, macrophages, and fibroblasts target the extracellular matrix as well as parenchymal cells to produce a spectrum of inflammatory mediators and metabolites, membrane adhesion molecules, prothrombotic receptors, growth factors, and chemotactic agents. The inflammatory cascade in CVDs serves as a tissue repair mechanism, but the resulting valvular incompetence may favor further inflammation, which leads, in turn, to venous stasis and clinical manifestations (from varicosities to, ultimately, the occurrence of ulcers). Evidence is accumulating that surgery aimed at preventing venous reflux can also aid healing and prevent the recurrence of venous ulcers.6
At the very beginning of disease, the C0s patients (named phlebopathic patients by Andreozzi et al) may also have reflux in the subcutaneous trunks revealed by DS.7 The Italian team, on its side, did not find any reflux in such patients upon DS investigation, but they detected a localized valve dysfunction with flaps or prolapse.8 A recent study has shown that incompetence can occur in human small superficial venous valves of C0 and C1 subjects, independently ofreflux within the great saphenous vein (GSV) and major tributaries.9 Reflux in these micro venous valves may play a role in the appearance of venous symptoms, but we are presently unable to perform this assessment.
In our experience, and although the diagnosis of phlebopathy is based primarily on the patient’s complaints, some objective signs such as venous hypervolemia could be identified.10,11,12 Venous hypervolemia is typified by its occurrence at the end of day and manifests by an increase in the volume of the calves, resulting in increased tightness when putting on long boots (also known as the “tight boot” symptom).10 The significant changes in the volume of the lower extremities, primarily the calves, are confirmed by plethysmography,10 as well as by an increase in the diameter of veins measured by repeating DS before and after prolonged orthostatic load.12
Since phlebopathy is thought to be not only a problem of venous tone, but also a question of valve dysfunction paired with venous inflammation, we searched for pharmacological treatments with a comprehensive mode of action that acts on the venous wall tone13 and is able to preserve venous valve structure.14 The beneficial anti-inflammatory and venoprotective actions of the micronized purified flavonoid fraction (MPFF)* has been evidenced in many studies of treatment efficacy in various forms of CVDs.13,15 Therefore, we chose MPFF for the present study that was designed to:
• Investigate the peculiarities of the GSV reflux in C0s (phlebopathic) patients after a prolonged time in a standing position (in the evening), before and after treatment with MPFF.
• Assess the associated venous symptoms and the quality of life of enrolled patients before and after treatment with MPFF.
*Also registered as MPFF at a dose of 500 mg, Alvenor, Ardium, Arvenum, Capiven, Detralex, Variton, Venitol
Material and Methods
This was an open-label study to analyze the efficacy of MPFF on transitory reflux and associated symptoms in phlebopathic patients assigned to the CEAP class C0s, En, An, Pn. Women complaining, at the end of the afternoon, of leg heaviness, pain, cramps and sensation of swelling in the calves, which were relieved after rest, were included in the study. The exclusion criteria were as follows: (i) history of vein surgery or sclerotherapy; (ii) history of venous thrombosis; (iii) presence of any visible venous signs on the lower extremities; and (iv) presence of concomitant diseases such as heart, lung, liver, or kidney insufficiency.
In addition to the routine clinical examination, all patients underwent DS of the lower extremities in the upright position twice a day: once in the morning (before 10 am) and once in the evening after normal physical effort and being in an upright position (after 6 pm). The reflux was considered abnormal if its duration was longer than 0.5 seconds.16 We also measured GSV diameter (mm) at the terminal segment in the evening and in the morning, and calculated the difference between the values for GSV diameter. The women with a detectable evening GSV reflux received MPFF (1000 mg once a day in the morning) for 2 months. Patients were not allowed to wear elastic compression stockings during the study period.
Duplex scanning was repeated after 2 months of MPFF treatment, as well as the measurement of GSV diameter, both in the morning and in the evening. Intensity of symptoms, such as heavy legs, pain, and cramps, was measured on a 10 cm visual analog scale (VAS) at baseline and at the end of MPFF treatment. The quality of life (QOL) was also assessed using the self-questionnaire ChronIc Venous Insufficiency Quality of life questionnaire-20 items (CIVIQ-20) at baseline and after 2 months of MPFF treatment. The global index score (GIS) of CIVIQ-20 ranges from 0 to 100. A GIS of 100 means the highest possible QOL, whereas a GIS of 0 means the worst possible QOL.
Statistical analysis was performed with Statistica 6.0 software. Mean values of parameters were calculated with a 95% confidence interval (95% CI). Group comparisons were done using a nonparametric Wilcoxon test.
Results
The study was performed in 2013 and included 41 women aged 21 to 57 years (mean age, 35.4±15.1 years) assigned to the CEAP class C0s, En, An, Pn. The intake of oral contraceptives and hormone replacement therapies within the past 5 years was reported in 22 women (54%). All women complained of heaviness and pain in the calves at the end of the afternoon. Night cramps and an increase in leg volume (the symptom of “tight boot”) were also reported in 21 and 28 women, respectively.
Duplex scanning investigation
A total of 15 of the 41 enrolled women had no reflux, either in the morning or in the evening. The remaining 26 women had no reflux in the morning, but presented with an evening GSV reflux. As for reflux extent, 2 had an axial reflux and 24 had a segmental reflux, of which 11 were proximal and 13 were medial. Regarding reflux type, it was found to be commissural in 22 and intervalvular in 4 women (Table I). It is important to note that in the 26 women with an evening reflux (Figure 1), the morning DS investigation after a night’s rest did not show any reflux.
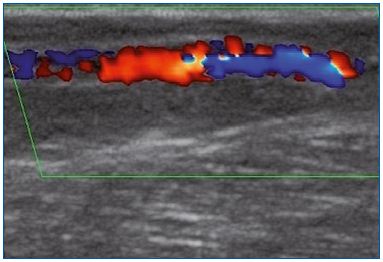
Figure 1A. Longitudinal section of the great saphenous vein at
the lower third of the thigh. Segmental reflux on duplex scanning
examination in the evening after being in a prolonged upright
position.
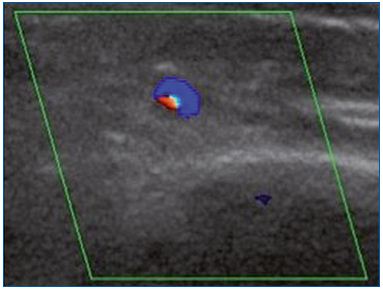
Figure 1B. Cross-section of the great saphenous vein at the lower
third of the thigh allowing an assessment of vessel diameter
(4.8 mm). Commissural reflux on duplex scanning examination
in the evening after being in a prolonged upright position.
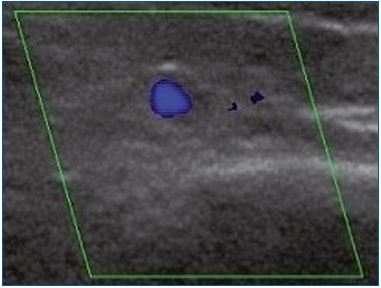
Figure 1C. Cross-section of the great saphenous vein in the
valve area at the lower third of the thigh allowing assessment of
vessel diameter (4.5 mm). Absence of reflux on duplex scanning
examination in the morning after rest.
Assessment of the great saphenous vein diameter
We compared the GSV parameters in the GSV terminal segment between women presenting with evening reflux and those without evening reflux. The evening GSV diameters and the difference in GSV diameter between evening and morning in the subgroup with reflux were higher (P<0.05) than in the subgroup without reflux (Table II).
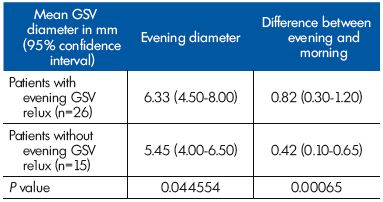
Table II. Parameters related to the great saphenous vein (GSV)
diameter at the GSV terminal segment in patients with and
without reflux (n=41).
Effect of MPFF treatment on evening reflux and on vein diameter
The evening reflux was eliminated in 22 of the 26 women. The 22 women with no longer evening reflux presented with a commissural reflux at baseline. In the 4 women who had intervalvular reflux, the extent of reflux slightly decreased, but this was not statistically significant. The evening vein diameter decreased to normal valves after MPFF treatment (Table III).
Effect of MPFF treatment on symptom relief and quality of life
The change in the intensity of associated venous complaints, such as heaviness, pain, and night cramps, in patients before and after treatment is shown in Figure 2. Interestingly, evening leg heaviness, pain, and cramps significantly decreased after 2 months of MFPP treatment. A parallel improvement in their QOL was seen with CIVIQ-20: from 57.97±7.63 at baseline, the GIS increased to 69.64±8.65 after a 2-month treatment with MPFF (P=0.000001).
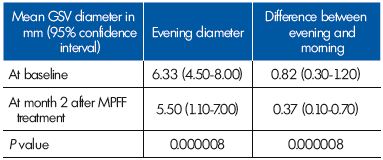
Table III. Parameters related to the great saphenous vein (GSV)
diameter in the terminal segment in women with evening reflux
(n=26) at baseline and after a 2-month therapy with MPFF.
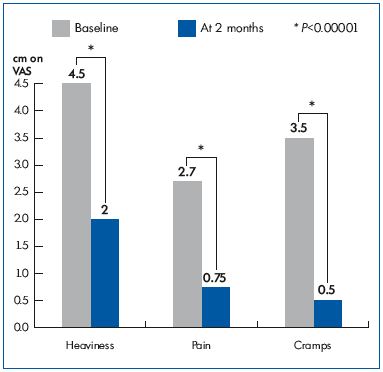
Figure 2. Symptom intensity on the 10 cm visual analog scale
(VAS) before (baseline) and after MPFF treatment (at 2 months).
Discussion
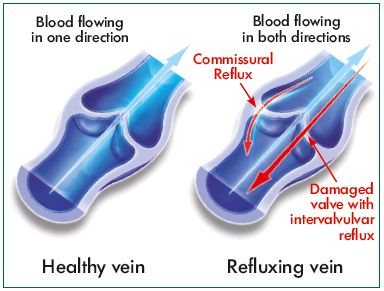
Transitory segmental incompetence occurred in almost half of the C0s patients of the present study. Such reflux was not present in the morning after a night’s rest because it is not related to destruction of valves. In addition, the majority had a transitory reflux of the commissural type. Commissural reflux, through one or both spaces between the valve leaflets of the lower third of the thigh in young patients in the standing position, has previously been demonstrated by Shadeck.17 Although it hemodynamically mimics pathological reflux as the duration greatly exceeds 0.5 seconds, it remains localized to the valve with no distal extension and fully meets the definition of reflux through a healthy valve demonstrated in the GSV terminal segment. Shadeck considers that this might reflect no more than slow closure of the commissural space. For Van Cleef, such transitory incompetence affecting valves with a normal appearance is the reflection of a dilatation of the perivalvular venous ring without significant damage to the valve apparatus itself.18
Two of the enrolled women presented with intervalvular reflux, which is usually associated with incompetent valve leaflets that are damaged and no longer function. Such reflux is considered pathologic. The MPFF treatment slightly decreased reflux length in these 2 patients even though this improvement was not statistically significant.
In our study, transitory reflux was associated with an increased GSV diameter in the evening compared with the morning. Increased GSV diameter participates to the occurrence of a reflux because it prevents valve leaflets from closing properly. Previous studies have correlated increasing GSV diameter with increasing CEAP clinical classification, but failed to demonstrate that decreasing GSV diameter improves QOL.19 On the other hand, the presence of venous symptoms greatly worsens the QOL of CVDs patients.20 In our study, improvement in QOL might not be related to a decrease in GSV diameter by MPFF, but rather to symptom improvement.
MPFF significantly decreased GSV dilatation, thereby facilitating valve closure. The improvement in the viscoelastic properties of GSV by MPFF was evidenced in earlier works.21 In the case of MPFF, venous tone is reinforced by modulation of noradrenergic signaling through reduced norepinephrine metabolism.21,22
The mechanism that triggers dilatation of the perivalvular area is currently unknown. For Shadeck, there is no demonstrated evidence that transitory reflux heralds irreversible valve incompetence,17 and yet, Schultz- Ehrenburg has previously shown that a preclinical venous reflux in children not only precedes the occurrence of truncal varicose veins (VV), but also that such reflux represents a 30% risk (95% CI, 13% to 53%) of developing VV disease within 4 years.23 It can be speculated that, in predisposed subjects, such as the C0s ones, a prolonged standing position and subsequent prolonged pooling of blood causes distortion of the venous valves and leads to reverse venous flow. Such flow disturbances can initiate and maintain an inflammatory reaction, which is responsible for the associated venous symptoms seen in the C0s subjects. Inflammatory events occur largely in response to abnormal venous flow and are important in causing the adverse changes in both the venous valves and vein wall that, in turn, become irreversible with time.4 One of the most peculiar features of the mode of action of oral MPFF is its ability to protect valve morphology in models of venous hypertension. Such protection is related to attenuation, by MPFF, of various elements of the inflammatory cascade, notably the endothelium-leukocyte interaction.13,14 This could explain the results found in the present study.
Despite the fact that hormonal influence on the occurrence of CVDs in women is disputed, sex hormones are included in the risk factors traditionally cited as contributing to venous valve failure; these also include female sex, pregnancy, obesity, a standing occupation in women,24 and heredity.25 Receptors to estrogen and progesterone exist in the GSV wall.26 Progesterone is known to inhibit smooth muscle contraction, and is useful in preventing uterine contraction and spontaneous abortion. However, preventing vein wall smooth muscle contraction allows passive dilation of veins, and when a critical diameter is reached, a functioning venous valve becomes dysfunctional or incompetent.27 As half of a woman’s adult lifetime is under the influence of progesterone, which is exacerbated during pregnancy, it is no wonder that primary venous insufficiency is twice as common in women than in men.24
A recent study has shown that during the menstrual cycle, diameter and valve closure time of the lower limb veins increases. The authors hypothesized that such changes are mediated by the female sex hormones.28 However, the time of the menstrual cycle was not reported in our study and was one of its limitations. Reversible transient reflux occurring after prolonged standing is not consistently reproducible.29 Therefore, several DS evening assessments on consecutive days would have been desirable, but were not possible to perform due to limitation in study costs, reflecting another limitation of our study.
Conclusion
Our study has shown that active pharmacological treatment, such as treatment with MPFF, can protect valve structures from further damage, decrease GSV diameter, and eliminate transitory incompetence in active women who undergo prolonged periods of standing. This was significant in women with commissural transitory reflux. At the same time, MPFF relieved these women from venous symptoms and improved their quality of life.
REFERENCES
1. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248- 1252.
2. Guex JJ, Rabe E, Escotto SI, et al. The C0s patient: worldwide results from the Vein Consult Program. Phlebolymphology. 2012;19:182-192.
3. Andreozzi GM, Signorelli S, Di Pino L, et al. Varicose symptoms without varicose veins: the hypotonic phlebopathy, epidemiology and pathophysiology. The Acireale project. Minerva Cardioangiol. 2000;48:277-285.
4. Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
5. Schmid-Schönbein G. Inflammation and the pathophysiology of chronic venous insufficiency. Phlebolymphology. 2002;39:95-99.
6. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomized controlled trial. Lancet. 2004;363:1854- 1859.
7. Bogachev VYu. Hormone-induced phlebopathy. New problem of modern phlebology. Ang Vasc Surg. 2002;8(3):50-54.
8. Andreozzi GM. Prevalence of patients with chronic venous disease-related symptoms but without visible signs (described as C0s in the CEAP classification): the Italian experience. Phlebolymphology. 2006;13:28-35.
9. Vincent JR, Jones GT, Hill GB, van Rij AM. Failure of microvenous valves in small superficial veins is a key to the skin changes of venous insufficiency. J Vasc Surg. 2011;54:62S-69S.
10. Tsoukanov YuT. Local venous hypervolemia as a clinical pathophysiological phenomenon of varicose veins. Ang Vasc Surg. 2001;7:53-57.
11. Tsoukanov YuT, Tsoukanov AYu. Clinical assessment of phlebopathy severity by specification of leg heaviness symptom. Ang Vasc Surg. 2003;9:67-70.
12. Tsoukanov Yu T, Tsoukanov AYu, Bagenov VN. The effect of oral contraceptives on the orthostatic diameter of lower limb major veins and its correction. Ang Vasc Surg. 2008;14:75-77.
13. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Int Angiol. 2014;33:126-139.
14. Pascarella L, Lulic D, Penn AH, et al. Mechanisms in experimental venous valve failure and their modification by MPFF at a dose of 500 mg. Eur J Vasc Endovasc Surg. 2008;35:102-110.
15. Lyseng-Williamson KA, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
16. Labropoulos N. Cut point on normal and pathological values of reflux. Medicographia. 2008;30:157-162.
17. Schadeck M. Duplex scanning study of great saphenous veins in children: diameter, reflux and influence on therapy [in French]. Phlébologie. 1996;49:413- 418.
18. Van Cleef JF, Hugentobler JP, Desvaux P, Griton P, Cloarec M. Endoscopic study of reflux of the saphenous valve [in French]. J Mal Vasc. 1992;17:113-116.
19. Gibson K, Meissner M, Wright D. Great saphenous vein diameter does not correlate with worsening quality of life scores in patients with great saphenous vein incompetence. J Vasc Surg. 2012;56:1634-1641.
20. Launois R, Mansilha A, Jantet G. International psychometric validation of the Chronic Venous Disease quality of life Questionnaire (CIVIQ-20). Eur J Vasc Endovasc Surg. 2010;40:783-789.
21. I begbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology. 1997;48:45.
22. Gargouil YM, Perdrix L, Chapelain B, Gaborieau R. Effects of MPFF at a dose of 500 mg on bovine vessels contractility. Int Angiol. 1989;8:19-22.
23. Schultz-Ehrenburg U, Weindorf N, Matthes U, Hirche H. An epidemiologic study of the pathogenesis of varices. The Bochum study I-III [in French]. Phlébologie. 1992;45:497-500.
24. Criqui M, Denenberg JO, Bergan JJ, Langer RD, Fronek A. Risk factors for chronic venous disease: the San Diego population study. J Vasc Surg. 2007;46:331-337.
25. Boisseau MR. Chronic venous disease and the genetic influence. Phlebolymphology. 2014;21(2):100-111.
26. Mashiah A, Berman V, Thole HH, et al. Estrogen and progesterone receptors in normal and varicose saphenous veins. Cardiovasc Surg. 1999;7:327-331.
27. Bergan JJ. Venous valve incompetence: the first culprit in the pathophysiology of primary chronic venous insufficiency. Medicographia. 2008;30:87-90.
28. Asbeutah AM, Al-Enezi M, Al-Sharifi NM, et al. Changes in the diameter and valve closure time of leg veins across the menstrual cycle. J Ultrasound Med. 2014;33:803-809.
29. Schadeck M. Reflux in healthy valves of the great saphenous vein [in French]. Phlébologie. 1991;44:603-613.