Endovenous treatment of lower-limb varices by laser and radiofrequency
Chassieu – France
SUMMARY
Endovenous treatment of varicose veins is an old technique, but new procedures using both radiofrequency and laser have markedly revived interest in it. In effect, the thermal energy these procedures generate and deliver to the venous wall results in vein wall fibrotic retraction and its subsequent occlusion. Details are given on the material and techniques used, including the difficulties, incidents, and accidents that can be encountered during the procedure. The indications and contraindications concerning endovenous treatment of varicose veins are still debatable. Assessment of results remains difficult, particularly in laser procedures, since the material and technique used is variable and the techniques nonstandardized. Besides long-term results are not yet available.
INTRODUCTION
Varicose veins are subcutaneous veins that are permanently dilated, exhibit vessel wall alterations, and have a diameter greater than 3 mm when the patient is standing. They are usually tortuous, but the fact that they are a site of reflux is rarely mentioned.
Endovascular treatment means any therapeutic procedure carried out from the lumen of the vein that results in occlusion of the diseased vein, without anatomical excision, excluding sclerotherapy. Until the last few years, endovascular treatment of varicose veins had only seen limited development.
Principle and mode of action of radiofrequency (RF)
and endovenous laser treatment (EVL)
Radiofrequency generates controlled thermal energy that raises the temperature of the vascular wall. It destroys the intima and causes contraction and thickening of the collagen fibers within the adventitia and especially the media (Figures 1a, 1b, 1c).
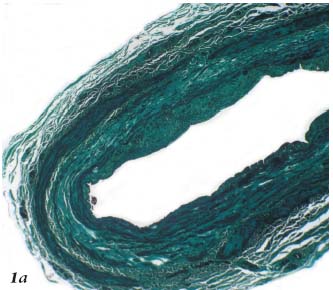
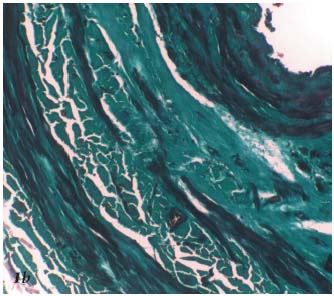
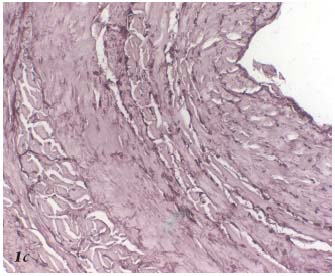
Figure 1a. Histopathology of the saphenous vein after Closure.
Coagulation necrosis of the intima and media. Trichrome-light
green stain.
Figure 1b. Histopathology of the saphenous vein after Closure. The
myocytes of the media are stretched and flattened within the necrotic
area. Trichrome-light green stain.
Figure 1c. Histopathology of the saphenous vein after Closure. The
elastic fibers are fragmented. Orcein.
The diameter of the vein is thus greatly reduced by contraction and thickening of collagen fibrils and also by the spasm induced by the increased temperature. These phenomena cause secondary fibrous changes, which are usually gradual, and result in the occlusion of the venous lumen (Figure 2). Thrombus formation is reduced to a minimum, since the procedure is performed on a vein containing no blood, eliminating the risk of recanalization by thrombolysis.
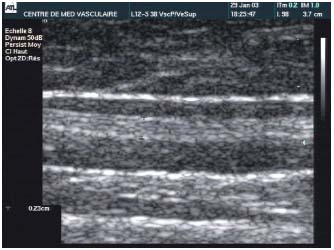
Figure 2. Fibrous transformation of the main body of the great
saphenous vein. Doppler sonography checkup 4 years after the
Closure procedure.
Radiofrequency produces this controlled thermal effect through a generator connected to a catheter. The catheters used have bipolar electrodes that deliver a temperature of 85°C at their tip (Figure 3). This temperature increase is obtained over a cylindrical zone, 6 to 8 mm long. The diffusion of this heat depends on the distance from the electrode, the temperature decreasing progressively with the distance from the point of contact between the catheter and the vein, falling to values in the region of 43°C at a distance of 2 mm. This is why the thermal energy is diffused continuously.
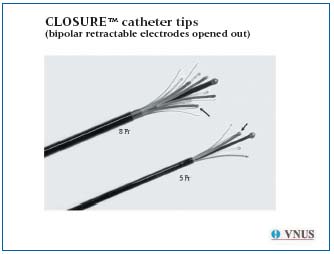
Figure 3. Tip of the Closure 5 and 8 F catheters.
Lasers. Laser treatment uses thermal energy, acting through three complex and successive stages:
– Conversion of light to heat by optical diffusion, which varies according to the medium in which the light is delivered. As Proebstle1,2 convincingly showed when studying the effects of the diode laser (810 nm, 940 nm, and 980 nm), the effect differs according to whether the light is delivered in normal saline, plasma, or blood. The laser energy, delivered into the blood with a 600-ìm fiber using successive pulses of variable length, creates bubbles of vapor generated by the hemolyzed blood. Indeed, the treated vein is not collapsed during this procedure. Three successive stages have been identified:
– Conversion of laser light into heat by optical diffusion. The volume heated, which in this case is blood, is termed the “primary heated volume.”
– Transfer of the heat by conduction into the surrounding tissues, ie, the venous wall. The bubbles transmit the thermal energy to the entire circumference of the internal venous wall, the “secondary heated volume.” The average temperature measured at the tip of the laser fiber is on average 729°C.3 The thermal effect is diffused weakly in blood: its penetration ability in the tissues is 0.3 mm2.
– The 3rd stage is thermochemical. It results in the destruction of tissue. The appearance on histopathology3 (Figures 4a, 4b, 4c) and ultrasound (Figure 5) of the treated vein is well documented. If applied continuously, the laser can cause perforation of the venous wall.3
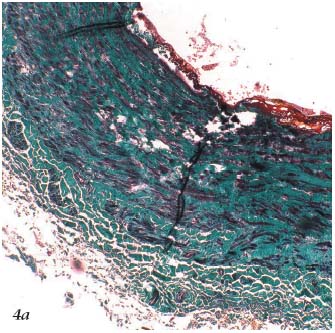
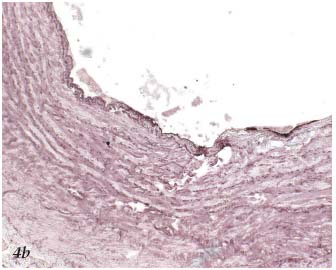
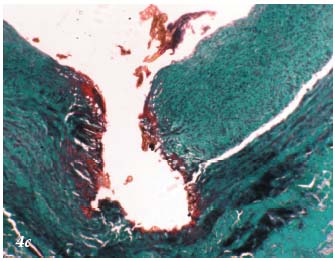
Figure 4a. Histopathology of the saphenous vein after laser
procedure. Trichrome-light green stain.
Figure 4b. Histopathology of the saphenous vein after laser
procedure. Coagulation necrosis involving the intima. Orcein.
Figure 4c. Histopathology of the saphenous vein after laser
procedure. Localized coagulation necrosis involving the intima.
Trichrome-light green stain.
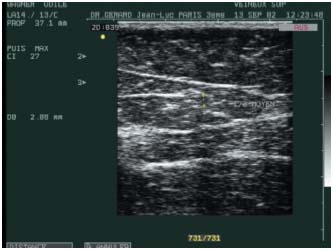
Figure 5. Atrophic appearance on Duplex sonography of the treated
vein, 6 months after laser treatment.
Equipment
Radiofrequency The equipment is currently marketed by a single company, and is called ClosureTM. Endovenous laser The equipment is currently marketed by several companies, providing diode lasers, which have supplanted the YAG laser.
Technique
1) RF and EVL procedures have a number of points in common. They are usually performed under local (tumescent in most cases) or locoregional anesthetic, and require:
– either a tiny surgical incision, with limited exposure of the distal part of the vein to be treated, which can be accessed using a phlebectomy hook, then opened by phlebotomy;
– or percutaneous puncture.
In practice the vein is accessed below the segment to be treated (usually at the knee for the great saphenous vein) after localization using Duplex sonography (DS). A straight or J-shaped metal guidewire or better still a hydrophilic guidewire is then passed along the vein, and its position is checked using DS. An introducer is then passed over the guidewire.
After the procedure, the (Closure) catheter or the fibercatheter unit (EVL) is withdrawn from the lumen of the vein. The cutaneous incision is closed and a compression bandage or an elastic stocking is put on the treated limb immediately.
2) ClosureTM procedure
The choice of catheter depends on the caliber of the vein to be treated: 6 F (1.7 mm) is used for veins with a diameter less than or equal to 8 mm, measured in the supine position, and 8 F (2.7 mm) for veins of more than 8 mm. The catheter is connected to the generator and to a heparinized saline infusion that is maintained throughout the procedure to prevent thrombus formation inside the catheter. The catheter is passed along the vein with the electrodes folded (Figure 6a) as far as the upper segment of the area to treat. The blood is expelled from the limb by applying an Esmarch bandage, with additional manual compression over the tip of the catheter, the patient lying with the legs higher than the head at an angle of about 20°. The electrodes are deployed to make contact with the wall of the vein (Figure 6b). The position of the catheter is then determined accurately (using ultrasound or fluoroscopy). It is important that the limb is not moved after this step. The various parameters are displayed on the screen of the generator: power (6 watts), temperature (85°C) and the duration of the procedure (999 s). Before commencing the procedure, the impedance is measured. The value displayed should be greater than or equal to 200 ohms, indicating that there is adequate contact between the electrodes with the venous wall. The treatment is then started. The catheter, with its electrodes deployed, is slowly drawn back from the proximal to the distal end of the area to be treated (Figures 6c, d) at a speed of 2 to 3 cm/minute and the various parameters are monitored constantly. The maintenance of a constant temperature (85±3°C) determines the speed at which the Closure catheter can be advanced.
As mentioned above, two localization techniques may be used. The choice is usually made on the basis of the local availability of these investigations. Radiological techniques are rarely favored.
Duplex sonography (DS)4 is the most commonly used investigation. Initially, it is used while entering the catheterized vein and later to monitor the progression of the catheter and its final position before radiofrequency is delivered, and finally to check the efficacy of the procedure after the intervention (absence of flow). The use of DS requires the presence of an operator who is experienced in this investigation, but it is faster than radiological mapping and is easy to repeat.
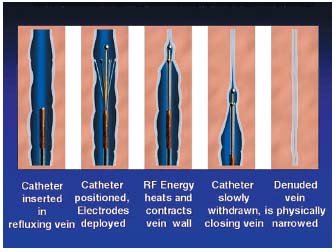
Figures 6 a, b, c, d. Closure procedure.
3) Laser procedures
Initially, the length of the vein to be treated is marked on the catheter using a Steri-strip or Steri-stip. It corresponds to the distance between the point of introduction and the point corresponding to the upper limit of the vein to be treated (Figure 7). Similarly, the length of the catheter plus 2 cm is marked on the laser fiber. The catheter is then introduced into the lumen of the vein and moved along the guidewire using the introducer, which is left in place after any verification of reflux and rinsing with normal saline. Its tip must be positioned 4 cm below the upper limit of the vein to be treated (Figure 8), its position is easily checked by DS, whereas the laser fiber is difficult to identify. The guidewire is then withdrawn and the laser fiber connected to the generator in standby position. The laser fiber is then introduced into the lumen of the catheter and pushed in until its tip (the sighting beam) is visible, which happens as soon as it emerges from the catheter as it is luminescent (Figure 9). The tip of the fiber is therefore positioned 2 cm below the vein to be treated (Figure 8). The fiber and the catheter are locked together. If the local anesthetic that was given at the point of introduction is used, it is then adminis- tered along the entire course of the vein to be treated. Tumescent anesthesia is the most common method. Everyone present in the theater wears protection glasses. The fiber-catheter unit is then withdrawn and disconti- nuous laser pulses are fired along the vein (Figure 10).
Figure 7. Laser procedure: the distance between the point where the
fiber was introduced and the upper part of the vein to be treated is
measured and marked on the laser fiber.
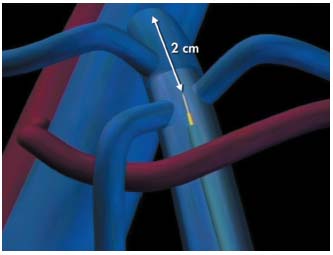
Figure 8. The tip of the laser fiber is positioned 2 cm below the
saphenofemoral junction. It protrudes from the catheter by 2 cm.
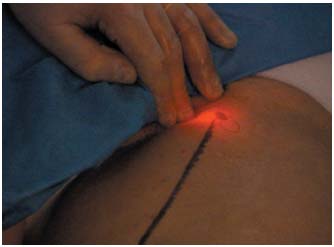
Figure 9. Laser procedure: the laser fiber is easily identified when it
protrudes from the proximal tip of the catheter.
Figure 10. Laser procedure: laser pulses are delivered as the fiber is
withdrawn.
The various parameters (duration of the pulses, distance between two pulses, etc) vary according to the type of laser used. However, certain types deliver laser energy continuously.
The sighting beam can be seen through the skin, which means that in most cases the tip of the laser fiber can be visualized during the procedure as it moves along the vein.
4) Additional procedures: when the treated vein is the trunk of the great or small saphenous vein a number of further procedures can be performed.
Saphenofemoral junction ligation. This is no longer performed in the Closure technique because a study5 showed that the results were equivalent with or without saphenofemoral junction ligation. It can be associated with the laser procedure6 or not.2,7-9
Phlebectomy or sclerotherapy of collateral vessels. In the Closure technique, phlebectomy using mini-incisions along the collateral or tributary veins is usually combined with treament for venous insufficiency of the main vessel. As a rule, sclerotherapy is used postoperatively.
In EVL techniques, phlebectomy was performed in one series with a laser fiber.6 In the others, the diseased collaterals were treated during the same operation by multiple-incision phlebectomy8 or later by sclerotherapy.2,9
Intraoperative problems, incidents and accidents. Very few were reported in the various series analyzed.2,6,7,10,11
Postoperative complications. These are presented in Tables I, II, and III.
Contraindications. There are some contraindications to the Closure technique: presence of thrombus in the vein to be treated or extreme tortuous vein. A very superficial GSV is not a contraindication, because the risk of cutaneous burning can be avoided.
For EVL, the various companies mention no specific contraindications related to the morphology or diameter of the vein to be treated.
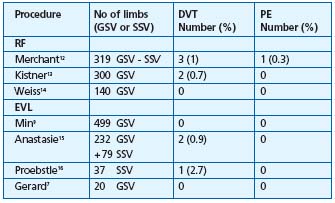
Abbreviations: ST = superficial thrombophlebitis; GSV = great saphenous vein;
SSV = small saphenous vein; DVT = deep vein thrombosis; PE = pulmonary embolism
Table I. Thromboembolic complications (excluding ST) after RF
and EVL.
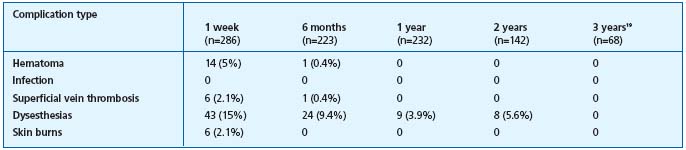
Table II. Complications following Closure.12,17
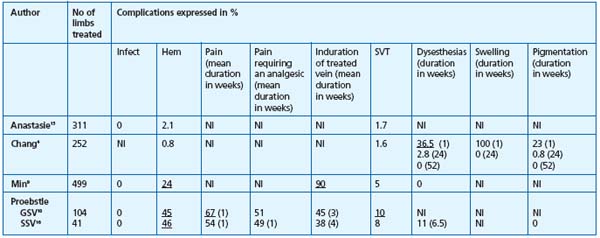
Abbreviations: Infect=Infection; Hem=Hematoma; SVT=superficial venous thrombophlebitis, NI=no information
Table III. Complications after EVL.
Therapeutic indications
1) Dependent on the anatomical or topographic localization of the varicose veins to be treated. The GSV is generally treated by surgery, and is usually limited to the portion situated above the knee due to the risk of damage to the saphenous nerve in the crural segment. This same neurological risk means that the SSV has been treated less frequently using endoluminal procedures, but one series of SSVs was nevertheless treated by diode laser.16 Where there is no venous insufficiency of the saphenofemoral junction or the main trunk, the collaterals of varicose saphenous veins can also be treated using EVL.19
2) Dependent on the clinical context. In theory they are identical to those for conventional surgical treatment of varicose veins.
Results
The results of surgery on varicose veins are difficult to judge at the clinical level. We will discuss briefly the main reasons for this.
Depending on the series, treatment of the saphenous trunk was either supplemented or not supplemented during the initial procedure by high ligation or/and stab avulsion of the branches. Whether or not these adjunctive procedures had influence on the treatment results is not clear.
Similarly, it is difficult to determine the importance of the additional procedures performed or not performed during the follow-up period.
Finally, the result can be judged on the basis of different criteria: clinical (symptoms, signs, quality of life) or hemodynamic criteria. However, not all of these criteria can be quantified easily.
Traditionally, the result is assessed in terms of:
The symptoms, which remain a subjective element. To quantify each symptom, a descriptive term can be used, generally 3 or 4 adjectives: absent, moderate, (significant), severe. The same scale may be used for each symptom.
A visual, analogue pain scale appears, however, to be more quantitative.
Whatever tool is used, it must be remembered that the so-called “venous” symptoms do not correlate well with presence or absence of varicose veins.20
Clinical signs. There are different classifications; the most commonly used classification is the CEAP,21 but it is relatively unsuitable for varicose veins when there is no skin or subcutaneous change. The European phlebological file22 recently renamed Computerized Venous Registry is probably a more precise tool. However, if the esthetic result is considered above all, it has been shown that this result is assessed differently by the physician and the patient.23
The venous clinical severity scores incorporating both signs and symptoms are certainly more suitable for judging the effectiveness of a treatment.24 This score was validated,25 but it seems more suitable for severe forms of chronic venous disease, ie, chronic venous insufficiency.26
– Quality of life is measured by generic and specific questionnaires.27 Quality of life has already been used to estimate the results of conventional varicose vein surgery at 2 years.28
All of the comments just made concerning the difficulty of judging the results clinically are also valid for conventional surgery and sclerotherapy.
The results judged by means of the duplex US investigation, namely complete obliteration of the vein treated by EVL or RF appear equivalent percentage-wise, of the order of 90% at 3 years. In Merchant’s series, we note a satisfying correlation between the clinical result and duplex US when the result is judged by the physicians, undoubtedly influenced by their knowledge of duplex US (Table IV).
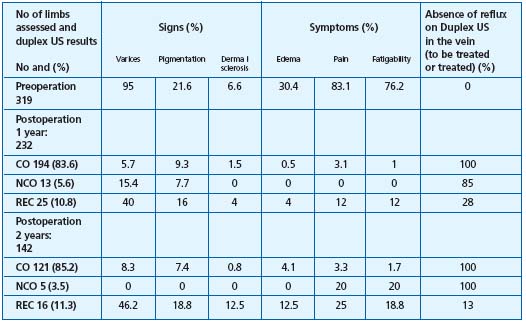
Table IV.
Radiofrequency
clinical results
and duplex US.12
Abbreviations:
CO: complete occlusion, NCO: near
complete occlusion (patent<5 cm),
REC; recanalization (patent >5 cm
It must also be noted that in the absence of long-term results for the endoluminal methods, we do not know what the future holds, clinically speaking, for patients with a patent SFJ stump (patient listed near complete obliteration (NCO) following RF). It is known only that at 2 years, there is no significant difference between the complete obliteration and NCO groups).12
Finally, we do not currently have at our disposal controlled studies allowing us to judge mid-term or long-term endoluminal surgery versus conventional surgery.
However, indirect comparison is possible using data from different studies as a reference, in which patients have benefited from systematic preoperative duplex US. For this purpose, we have the Rutgers29 series, which analyzed at 3 years the results for 69 limbs treated by crossectomy (high ligation)+ stripping of the trunk±phlebectomy of the branches. It appears that there is no significant difference in terms of clinical results with RF used alone where the results published provide 3 years of hindsight.17
One point is worth highlighting: the absence of the phenomena of neovascularization, in particular at the level of the saphenofemoral and saphenopopliteal junctions following endoluminal surgery. It is known that this phenomenon, which occurs frequently after conventional surgery, plays an important role in the incidence of recurrence.30-35
It is difficult to determine whether not using high ligation can alone explain the absence of neovascularization. It is also possible that maintaining the patency of the saphenous termination tributaries plays a favorable role in that they drain physiologically.36,37
CONCLUSIONS
The following conclusions may be drawn after analysis of the publications on RF and EVL:
Many articles have been written on endovenous laser and venous ablation using radiofrequency. Whereas the RF procedure has been standardized, the protocols for EVL vary because of the diversity of the equipment used.
The time to resumption of normal activities and the length of the convalescence period are considerably shorter after endoluminal procedures. The type and number of complications associated with these interventions are well documented. They appear to be transient and mild.
The medium-term clinical results for RF are better documented than those of EVL. In terms of hemodynamics, ablation of the treated vein is identified by Duplex sonography in 90% of cases after 3 years for both procedures.
The absence of controlled long-term studies compared with sclerotherapy and conventional surgery means that grade A or B recommendations cannot currently be formulated for them.

REFERENCES
2. Proebstle TM, Lehr HA, Kargl A, Espinosa- Klein C, Rother W, Behtge S, Knop J. Endovenous treatment of the greater saphenous vein with a 940-nm diode laser: thrombotic occlusion after endoluminal thermal damage by laser-generated steam bubbles. J Vasc Surg. 2002;35:729-736.
3. Weiss R A. Comparison of endovenous radiofrequency versus 810-nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56-61.
4. Pichot O, Perrin M. Aspects échographiques de la jonction saphénofémorale après oblitération de la grande veine saphène par radiofréquence (Closure®). Phlébologie. 2002;55:329-334.
5. Chandler JG, Pichot O, Sessa C, Schuller- Petrovic S, Osse FJ, Bergan JJ. Defining the role of extended saphenofemoral junction ligation: a prospective comparative study. J Vasc Surg. 2000;32:941-953.
6. Chang CJ, Chua JJ. Endovenous laser photocoagulation (EVLP) for varicose veins. Lasers in Surgery and Medicine. 2002;31:257-262.
7. Gerard J-L, Desgranges P, Becquemin J-P, Desse G, Mellière D. Peut-on traiter les grandes saphènes variqueuses par laser endoveineux en ambulatoire? Résultats à 1mois d’une étude de faisabilité sur 20 patients en salle de consultation. J Mal Vasc. 2002;27:222-225.
8. Guex JJ, Min RJ, Pittaluga P. Traitement de l’insuffisance de la grande veine saphène par photo coagulation laser endoveineuse: techniques et indications. Phlébologie. 2002;55:239-243.
9. Min RJ, Zimmet SE, Isaacs MN, Forrestal MD. Endovenous treatment of the incompetent greater saphenous vein. J Vasc Interv Radiol. 2001;12:1167-1171.
10. Lebard C, Zucarelli F. Intérêt de l’angiographie de la jonction saphénofémorale au cours de la destruction de la grande veine saphène par le système Closure. Phlébologie. 2002;55:263-268.
11. Navarro L, Boné C. L’énergie laser intraveineuse dans le traitement des troncs veineux variqueux : rapport sur 97 cas. Phlébologie. 2001;54:293-300.
12. Merchant RF, DePalma RG, Kabnick LS. Endovenous obliteration of saphenous reflux: a multicenter study. J Vasc Surg. 2002;35:1190-1196.
13. Kistner RL. Endovascular obliteration of the greater saphenous vein: The Closure procedure. Jpn J Phlebol. 2002;13:325-33.
14. Weiss RA, Weiss MA. Controlled radiofrequency endovenous occlusion using a unique radiofrequency catheter under duplex guidance to eliminate saphenous varicose vein reflux: A 2-year follow-up. Dermatol Surg. 2002;28:38-42.
15. Anastasie B, Celerier A, Cohen-Solal G, Anido R, Boné C, Mordon S , Vuong PN. Laser endoveineux. Phlébologie. 2003;56:369-382.
16. Proebstle TM, Doendue G, Kargl A, Knop J. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: Early results. Dermatol Surg. 2003;29:357-361.
17. Perrin M. Varices des membres inférieurs traitées par radiofréquence (CLOSURE®). Contrôle annuel des résultats : un suivi sur 3 ans. Phlébologie. 2004;57:69-73.
18. Proebstle TM, Gül D, Lehr HA, Kargl A, Knop J. Infrequent early recanalization of greater saphenous vein after endovenous laser treatment. J Vasc Surg. 2002;38:511-516.
19. Navarro L, Min RJ, Boné C. Endovenous laser: a new minimally invasion method of treatment for varicose veins–preliminary observations using an 810 nm diode laser. Dermatol Surg. 2002;27:117-122.
20. Bradbury A, Evans CJ, Allan PL, Lee AJ, Ruckley CV, Fowkes FGR. What are the symptoms of varicose veins? Edinburgh vein study cross sectional population survey. BMJ. 1999;318;353-356.
21. Porter JM, Moneta GL, and an international consensus committee on chronic venous disease. Reporting standards in venous disease: an update. J Vasc Surg. 1995:27:635-645.
22. Uhl JF, Cornu-Thénard A, Carpentier PH, Chleir F. Le Dossier Médical Phlébologique Européen (DMPE) : Son fonctionnement et ses applications passées, présentes et futures. Phlébologie. 2002;55:121-125.
23. Jakobsen BJ. The value of different forms of treatment for varicose veins. Br J Surg. 1979;66:182-184.
24. Rutherford RB, Padberg FT, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000; 31:1307-1312.
25. Meissner MH, Natiello C, Nicholls SC. Performance characteristics of the venous clinical severity score. J Vasc Surg. 2002;36:889-895.
26. Perrin M, Dedieu F, Jessent. Une appréciation des nouveaux scores de sévérité de la maladie veineuse chronique des membres inférieurs. Résultats d’une enquête auprès d’angiologues français. Phlébologie. 2003;56:127-136.
27. Perrin M. Qualité de vie et chirurgie veineuse. Phlébologie. 2003;56:151-155.
28. MacKenzie RK, Paisley A, Allan PL, Lee AJ, Ruckley CV, Bradbury AW. The effect of long saphenous vein stripping on quality of life. J Vasc Surg. 2002;35:1197-1203.
29. Rutgers PH, Kitslaar PJEHM. Randomized trial of stripping versus high ligation with sclerotherapy in the treatment of the incompetent greater saphenous vein. Am J Surg. 1994;168:311-315.
30. Glass GM. Neovascularization in recurrence of the varicose great saphenous vein following transection. Phlebology. 1987;2:81-91.
31. Darke SG. The morphology of recurrent varicose veins. Eur J Vasc Surg. 1992;6:512-517.
32. De Maesener MG, Ongena KP, Van den Brande F, Van Schill PE, De Hert SG, Eyskens EJ. Duplex ultrasound assessment of neovascularization after saphenofemoral or sapheno-popliteal junction ligation. Phlebology. 1997;12:64-68.
33. Jones L, Braithwaite BD, Selwyn D, Cooke S, Earnshaw JJ. Neovascularisation is the principal cause of varicose vein recurrence: results of a randomized trial of stripping the long saphenous vein. Eur J Vasc Surg. 1996;12:442-445.
34. Nyamekye I, Shepard NA, Davies B, Heather BP, Earnshaw JJ. Eur J Vasc Endovasc Surg. 1998;39:412-415.
35. Van Ryj AM, Jiang P, Solomon C, Christie RA, Hill GB. Recurrence after varicose vein surgery: a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography. J Vasc Surg. 2003:38:935-943.
36. Pichot O, Kabnick LS, Creton D, Merchant RF, Schuller-Petrovic S, Chandler JG. Duplex ultrasound scan findings two years after great saphenous vein radiofrequency endovenous obliteration. J Vasc Surg. 2004;39:189-195.
37. Pichot O, Perrin M. Aspects échographiques de la jonction saphénofémorale après oblitération de la grande veine saphène par radiofréquence (Closure®). Phlébologie 2002;55:329-334.
