Endovenous laser ablation
Steven E. ZIMMET2,3
Austin, USA
ABSTRACT
Endovenous laser ablation (EVLA) is a less invasive alternative to vein stripping. Outcomes seem equal to, or better than, those with stripping, with better quality of life scores in the post-operative period. EVLA has been shown to correct or significantly improve hemodynamic abnormality in patients with chronic venous insufficiency (CVI) with superficial venous reflux. Early reports suggest that endovenous ablation techniques, in contrast to surgical stripping, are associated with a low incidence of neovascularization.
A variety of wavelengths are being used to perform EVLA. While the initial chromophore is water or hemoglobin, depending on the wavelength used, carbon appears to be a secondary but key chromophore that is probably independent of wavelength.
The application of the principles of tumescent anesthesia to venous treatments, along with the development of endovenous ablation techniques, offer the possibility of treating the vast majority of patients with varicose veins in-office without general anesthesia or surgical incisions, while at the same time maximizing outcomes and minimizing recurrence.
INTRODUCTION
Saphenous vein reflux is the underlying primary abnormality in the majority of cases of superficial venous insufficiency. Thus, approaches to dealing with saphenofemoral junction and saphenous truncal incompetence have dominated the thinking of phlebologists. Trendelenburg described saphenofemoral junction ligation alone, without stripping of the incompetent saphenous vein, in the 1890s. The advantages of ligation alone over ligation and stripping, which are still extolled today,1 include preservation of the saphenous trunk for possible future use as a bypass graft2 and avoidance of saphenous nerve injury.3 High ligation by itself is less invasive, quicker and simpler to perform, and associated with an easier recovery when compared to vein stripping. While it is true it routinely “spares” the saphenous trunk,4 the use of a diseased saphenous vein as a conduit has been associated with an increased risk of graft failure.5 Most importantly, there is no longer any question that high ligation alone usually results in persistent reflux in the saphenous trunk.6,7 Varicose recurrence is significantly reduced7-9 and the reoperation rate is 60% to 70% less if the saphenous vein is stripped compared with ligation alone.10,11 Also, after ligation alone, recurrence or residual communication with the junction in the groin was found in 80% of patients, while 34% also had mid-thigh perforator incompetence via the unstripped great saphenous vein (GSV).12 As Neglen concluded, stripping of the GSV of the thigh is essential to minimizing recurrence that is caused by redevelopment of incompetent communication with the saphenofemoral confluence, and due to thigh perforator incompetence.13 Simply put, the shortcomings of ligation alone outweigh its advantages.
It is important to note that recurrence is common even after ligation and stripping of the saphenous vein. While inadequate surgery of the saphenofemoral junction and progression of disease are mechanisms that explain some cases of recurrence, another important mechanism is neovascularization around the junction after venous surgery.11,14 In fact, neovascularization has been reported as the principle cause of recurrence,9 with neovascular channels of variable size, number, and tortuosity accounting for the reflux to recurrent varicosities in the majority of cases.15 Though some have expressed doubt as to the veracity of true neovascularization, there is clear histological evidence that neovascularization is a cause of recurrent varicose veins.16 Early reports suggest, in contrast, that endovenous ablation techniques are associated with a very low incidence of neovascularization. 17 It may be that the development of neovascularization is largely prevented by avoiding groin dissection and by preserving venous drainage in normal junctional tributaries.18,19
EVLA, like radiofrequency ablation and foam sclerotherapy, is a less invasive alternative to vein stripping. EVLA is indicated in an ambulatory patient with great, small or accessory saphenous vein reflux with surface varices and/or symptoms or complications related to superficial venous insufficiency. EVLA is routinely performed using dilute local anesthesia, with or without supplemental oral anxiolytics, in an office setting. Generally taking 30-60 minutes to perform, procedure times are dependent on the length of segment treated, experience of the operator, and whether ancillary procedures, such as ambulatory phlebectomy, are done. Regardless of how underlying saphenous incompetence is treated, ancillary treatments are typically needed to treat residual varices (Figure 1).
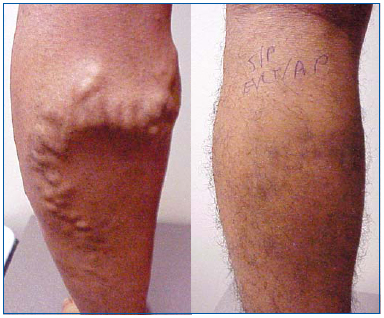
Figure 1. 26 y/o male before and 1 month after endovenous laser ablation of the great saphenous vein and ambulatory phlebectomy of varicose tributaries.
EFFICACY
Short- and mid-term studies of EVLA, regardless of wavelength used, seem remarkably consistent, typically reporting ablation of refluxing saphenous veins in 90% or more of cases.18,20-23 EVLA of the saphenous vein has been shown to correct or significantly improve the hemodynamic abnormality and clinical symptoms of chronic venous insufficiency (CVI) in Clinical, Etiological, Anatomical, Pathophysiological (CEAP) clinical class 3-6 patients with superficial venous reflux.24,25 Outcomes seem equal to or better than those of stripping, with better quality of life scores in the postoperative period compared to stripping.20,25-27 High patient satisfaction rates have been reported.18,28,29 The total cost (cost of the procedure plus societal cost) of endovenous procedures is likely equal to or lower than that of surgery.27
Early data on treatment of the GSV with 810 nm and 940 nm devices suggest treatment failure is uncommon in patients treated with > 70 J/cm.30,31 A withdrawal rate of 2 mm/sec at 14 watts delivers 70 J/cm.
MECHANISM OF ACTION
The following wavelengths are in current use for EVLA: 810, 940, 980, 1064, 1319, 1320, and 2068 nm. It has been postulated that vein wall injury is mediated both by direct effect and indirectly via laser-induced steam generated by heating of small amounts of blood within the vein.32 Some have suggested that choice of wavelength greatly impacts results.23
The main chromophore of 1320 and 2078 nm lasers, at least initially, is water, while other wavelengths used for EVLA primarily target hemoglobin. Obviously it is imperative to thermally damage the vein wall adequately in order to obtain effective ablation. Some heating may occur by direct absorption of photon energy (radiation) by the vein wall as well as by convection from steam bubbles and conduction from heated blood. However, it is unlikely that these latter mechanisms account for the majority of impact on the vein. The maximum temperature of blood is 100°C. Laser treatment has been found to produce carbonization of the vein wall.33 Carbonization of the laser tip, which occurs at about 300°C, is noted following EVLA, and seems to occur regardless of the wavelength used.34 Carbonization of the laser fiber tip creates a point heat source and essentially reduces light penetration into tissue to zero.34,35 Mordon et al stated “The steam produced by absorption of laser energy by the blood is a tiny fraction of the energy necessary to damage the vein wall and cannot be the primary mechanism of injury to the vein with endovenous laser. The carbonization and tract within the vein walls seen by histology following endovenous laser can only be the result of direct contact between the laser fiber tip and the vein wall.”36 Dr Rox Anderson, director of The Wellman Center for Photomedicine at Massachusetts General Hospital, reported that carbon appears to be a secondary but key chromophore that is probably independent of wavelength (Figure 2).34 Note that fiber tip and shape may impact development of carbonization.37

Figure 2. Carbonization of 600-micron laser fiber tip secondary to endovenous laser ablation with a 1320 nm laser (Photo courtesy of Mark Forrestal, MD, FACPh).
TUMESCENT ANESTHESIA
EVLA should be performed under local anesthesia using large volumes of a dilute solution of lidocaine and epinephrine (average volume of 200-400 mL of 0.1% lidocaine with 1:1,000,000 epinephrine) that is buffered with sodium bicarbonate. This solution should be delivered either manually or with an infusion pump under ultrasound guidance so the vein is surrounded with the anesthetic fluid along the entire length of the segment to be treated (Figure 3).
The benefits of tumescent anesthesia for endovenous ablation include:
• anesthesia,
• separation of vein to be treated from surrounding structures,
• thermal sink, which reduces peak temperatures in perivenous tissues,
• vein compression, which maximizes the effect of treatment on the vein wall.
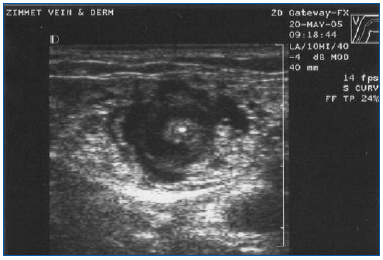
Figure 3. Transverse ultrasound image of tumescent anesthetic fluid surrounding centrally located great saphenous vein and laser fiber/sheath.
Although the maximum safe dosage of lidocaine using the tumescent technique for venous procedures is not well studied, a dosage of 35 mg/kg is a reasonable estimate.
Using these parameters, tumescent anesthesia in the context of liposuction has been shown to be extraordinarily safe. More information is available at http://www.liposuction.com/pharmacology/drug_inter act.php.
CONTRAINDICATIONS TO EVLA
Contraindications to EVLA technique are summerized in Table I.
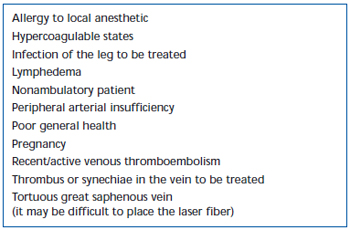
Table I. Contraindications to endovenous laser.
ADVERSE SEQUELAE
Short-term pain and ecchymoses have been commonly observed after EVLA. Intermittent-pulsed laser fiber pullback has been reported, in a retrospective review, to cause significantly greater levels of post-operative pain and bruising, compared with a continuous pullback protocol.38 Adding a short-stretch bandage for 3 days following intermittent mode EVLA substantially reduced patient-reported bruising and pain. Employing continuous mode pullback further reduced the severity of pain and bruising to such an extent that levels were similar to those reported by patients treated with radiofrequency ablation (Tables II and III). Preliminary reports suggest there may be some differences in postoperative course depending on wavelength used to perform EVLA.22,39 However, this is based on sparse data with short-term follow-up.
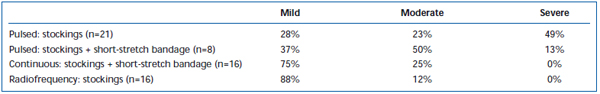
Table II. Patient-rated post-operative bruising 3-7 days following pulsed endovenous laser ablation (EVLA) with class II stockings, pulsed EVLA with stockings plus short-stretch bandage, continuous mode EVLA with stockings and short-stretch bandage, and radiofrequency ablation with stockings.
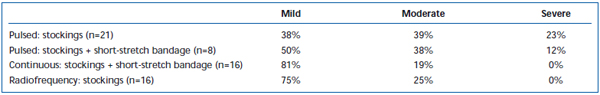
Table III. Patient-rated post-operative pain 3-7 days following pulsed endovenous laser ablation (EVLA) with class II stockings, pulsed EVLA with stockings plus short-stretch bandage, continuous mode EVLA with stockings and short-stretch bandage, and radiofrequency ablation with stockings.
PERIVENOUS THERMAL INJURY
Mean peak intravascular temperatures during EVLA (goat jugular vein, 12 watts, 1-second pulses, 1-second intervals), measured flush with the laser tip, averaged 729°C, while those 4 mm distal to the tip averaged 93°C.40 However, the risk of collateral thermal injury depends on perivenous tissue heating, not intravascular temperature.
Collagen has been noted to contract at about 50°C, while necrosis occurs between 70°C and 100°C.41 The extent of thermal injury to tissue is strongly dependent on the amount and duration of heat the tissue is exposed to. Henriques and Moritz investigated the time-temperature response for tissue exposed to up to 70°C.42 They found that skin could withstand temperature rises for very short exposure times, and that the response appears to be logarithmic as the exposure times become shorter. For example, an increase in body temperature to 58°C will produce cell destruction if the exposure is longer than 10 seconds. Tissues, however, can withstand temperatures up to 70°C if the duration of the exposure is maintained for less than 1 second. Li et al reported that heating endothelial cells to 48°C for 10 minutes did not induce cell death.43 They also found that osteoblasts, after exposure for 10 minutes or less at 45°C, underwent transient and reversible changes. Another study found reversible tissue damage to the hind limb of mice after submersion in a waterbath at 44°C.44
A recent study measured peak temperature at the outer vein wall during EVLA in a live pig ear vein and in exposed hind limb veins.45 EVLA settings ranged from 8 watts (1-2 second pulse durations), 10 watts (1-1.5 second pulse duration), 12 watts (0.5-1.5 second pulse duration) to 15 watts (0.5-1.0 second pulse duration), with and without tumescent anesthesia. Results demonstrate that peak temperatures ranged from 34.6°C to 49.1°C as a function of joules delivered, with lower peak temperatures obtained when tumescent fluid was present (Figure 4).
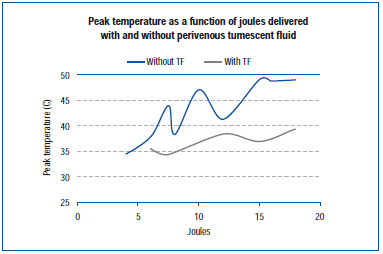
Figure 4. Adapted from Zimmet SE, Min RJ. Temperature changes in perivenous tissue during endovenous laser treatment in a swine model. J Vasc Interv Radiol. 2003;14:911-915 (ref 45).
Peak temperature measured during EVLA (63 patients, 980 nm, 15 watts, 1.5 sec pulses) at the outer vein wall in humans, 3 cm below the saphenofemoral junction, was 40.9°C and 49.8°C with and without tumescent fluid, respectively.46 Similar results were reported from another human study during EVLA (12 patients, 810 nm, 12 W, 1 second pulses, 1 second intervals, tumescent technique), with peak temperatures of 43.3°C, 42.0°C, and 36.0°C at 3 mm, 5 mm, and 10 mm from the GSV, respectively. 47
There appears to be a very rapid fall-off in temperature over short distances during EVLA. This is probably in contrast to radiofrequency energy where microwave heating occurs around the tissue-electrode interface. The animal and human data suggest that peak perivenous temperatures generated during endovenous laser are unlikely to cause permanent damage to perivenous tissue in most situations. The peak temperature generated is reduced with the use of perivenous tumescent fluid. These findings seem to explain the very low reported incidence of nerve injury and skin burns following EVLA. One study, using a 1064 nm Nd: YAG laser, reported a very high incidence of paresthesia in 36.5% and skin burns in 4.8%.48 It should be noted that the amount of energy delivered was about three times higher than what is typically used and that treatment was done without tumescent anesthesia. Despite the low perivenous temperatures reported with EVLA, it is important to note that special caution is required when considering endovenous intervention in certain cases such as sciatic nerve varices.49,50
MAJOR COMPLICATIONS
Major complications following EVLA have been reported rarely. Rates of deep venous thrombosis (DVT), pooled from multiple series, are much lower than 1%.17,18,20,28 One group reported an incidence of thrombus extension into the femoral vein of 7.7%.51 However, in that study EVLA was done under general or spinal anesthesia. The fact that patients were not able to ambulate immediately postoperatively may have contributed to the high incidence of thrombus extension. There is a single report of an arteriovenous fistula that developed following EVLA of the short saphenous vein (SSV).52 One patient developed septic thrombophlebitis following EVLA combined with open ligation of perforators and stab phlebectomy.53 This resolved with antibiotic treatment and debridement.
ALTERNATIVE APPROACHES
EVLA and radiofrequency ablation (RFA)54,55 both appear to be effective treatments for saphenous incompetence. Advantages of EVLA over RFA include shorter procedure times and lower per treatment cost. Reported occlusion rates of EVLA generally are slightly higher than those obtained with RFA.56 Disadvantages of EVLA may include more bruising and discomfort in the early postoperative period, although this may be techniquedependent. Both techniques continue to undergo refinement, which will improve results. Both procedures, when performed using tumescent anesthesia, are associated with low complication rates.
Another emerging treatment for saphenous reflux is the use of foamed sclerosants delivered under ultrasound control. A gas, such as air or CO2, can be mixed with liquid detergent sclerosants to create foam, estimated to be about four times more potent than the liquid form of the same agent. Early results suggest this may be a valuable modality, as it is quick and inexpensive to perform with reported short- and mid-term success rates of about 75% to 90%. There are many variables regarding foam (eg, type and amount of gas, technique used to create foam, concentration and type of sclerosant used, volume injected, etc). There may be a higher risk of deep vein thrombosis following foam sclerotherapy compared with standard sclerotherapy. Proper technique is important to minimize the risk of this complication. Other side effects reported following foam sclerotherapy include visual and neurological events. There is a published report of stroke following foam sclerotherapy (20 mL polidocanol foam) in a patient with a 1.8 cm patent foramen ovale.57 Further experience and research with this modality will better delineate its risks as well as long-term efficacy.
CONCLUSION
Currently accepted principles of treatment of varicose veins serve to maximize outcomes from a hemodynamic and patient standpoint, while minimizing the risk of recurrence. Appropriate treatment of varicose veins begins with an accurate assessment of the underlying venous pathology and identification of sources of venous hypertension. The aims of treatment include elimination of the incompetent connections between the deep and superficial systems, as well as the obliteration of pathways of venous incompetence and incompetent varicose veins. It is evident that recurrence is reduced if the incompetent segment of the saphenous trunk is ablated.
Endovenous laser ablation is a less invasive alternative to vein stripping. Outcomes seem equal to or better than those of stripping, with better quality of life scores in the post-operative period. EVLA has been shown to correct or significantly improve hemodynamic abnormality in patients with chronic venous insufficiency with superficial venous reflux. Early reports suggest that endovenous ablation techniques, in contrast to surgical stripping, are associated with a low incidence of neovascularization.
The application of the principles of tumescent anesthesia to venous treatments, along with the development of endovenous ablation techniques, offer the possibility of treating the vast majority of patients with superficial venous insufficiency in-office without general anesthesia or surgical incisions, while at the same time maximizing outcomes and minimizing recurrence.
This paper was submitted in July 29, 2006.

REFERENCES
2. Large J. Surgical treatment of saphenous varices, with preservation of the main great saphenous trunk. J Vasc Surg. 1985;2:886-891.
3. Holme JB, Holme K, Sorensen LS. The anatomic relationship between the long saphenous vein and the saphenous nerve. Relevance for radical varicose vein surgery. Acta Chir Scand. 1988;154:631-633.
4. Rutherford RB, Sawyer JD, Jones DN. The fate of residual saphenous vein after partial removal or ligation. J Vasc Surg. 1990;12:422-426.
5. Panetta TF, Marin ML, Veith FJ, et al. Unsuspected preexisting saphenous vein disease: an unrecognized cause of vein bypass failure. J Vasc Surg. 1992;15:102- 110.
6. McMullin GM, Coleridge-Smith PD, Scurr JH. Objective assessment of ligation without stripping the long saphenous vein. Br J Surg. 1991;78:1139-1142.
7. Sarin S, Scurr JH, Coleridge Smith PD. Assessment of stripping the long saphenous vein in the treatment of primary varicose veins. Br J Surg. 1992;79:889-893.
8. Munn SR, Morton JB, Macbeth WA, McLeish AR. To strip or not to strip the long saphenous vein? A varicose vein trial. Br J Surg. 1981;68:426-481.
9. Jones L, Braithwaite BD, Selwyn D, Cooke S, Earnshaw JJ. Neovascularisation is the principal cause of varicose vein recurrence: results of a randomised trial of stripping the long saphenous vein. Eur J Vasc Endovasc Surg. 1996;12:442-445.
10. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg. 1999;29:589-592.
11. Winterborn RJ, Foy C, Earnshaw JJ. Causes of varicose vein recurrence: late results of a randomized controlled trial of stripping the long saphenous vein. J Vasc Surg. 2004;40:634-639.
12. Corbett CR, Runcie JJ, Lea TM, Jamieson CW. Reasons to strip the long saphenous vein. Phlebologie. 1988;41:766-769.
13. Neglen P. Treatment of varicosities of saphenous origin: comparison of ligation, selective excision, and sclerotherapy. In: Bergan JJ, Goldman MP eds. Varicose Veins and Telangiectasias: Diagnosis and Treatment. St. Louis, Mo, USA: Quality Medical Publishing; 1993:148-165.
14. Kostas T, Ioannou CV, Touloupakis E, et al. Recurrent varicose veins after surgery: a new appraisal of a common and complex problem in vascular surgery. Eur J Vasc Endovasc Surg. 2004;27:275-282.
15. Van Rij AM, Jones GT, Hill GB, Jiang P. Neovascularization and recurrent varicose veins: more histologic and ultrasound evidence. J Vasc Surg. 2004;40:296-302.
16. Nyamekye I, Shephard NA, Davies B, et al. Clinicopathological evidence that neovascularization is a cause of recurrent varicose veins. Eur J Vasc Endovasc Surg. 1998;15: 412-415.
17. Ravi R, Rodriguez-Lopez JA, Trayler EA, et al. Endovenous ablation of incompetent saphenous veins: a large single-center experience. J Endovasc Ther. 2006;13:244- 248.
18. Min RJ, Khilnani N, Zimmet SE. Endovenous laser treatment of saphenous vein reflux: long-term results. J Vasc Interv Radiol. 2003;14:991-996.
19. Bergan JJ, Rattner Z. Endovenous therapy-2005. Acta Chir Bel. 2005;105:12- 15.
20. Agus GB, Mancini S, Magi G. The first 1000 cases of Italian endovenous-laser Working Group (IEWG). Rationale, and long-term outcomes for the 1999-2003 period. Int Angiol. 2006;25:209-215.
21. Kalra M, Gloviczki P. Fifteen years ago laser was supposed to open arteries, now it is supposed to close veins: what is the reality behind the tool? Perspect Vasc Surg Endovasc Ther. 2006;18:3-8 (discussion 9- 10).
22. Kabnick LS. Outcome of different endovenous laser wavelengths for great saphenous vein ablation. J Vasc Surg. 2006;43: 88-93.
23. Goldman MP, Maritess M, Rao J. Intravascular 1320-nm laser laser closure of the great saphenous vein: a 6- to 12-month follow-up study. Dermatol Surg. 2004;30: 1380-1385.
24. Marston WA, Owens LV, Davies S, et al. Endovenous saphenous ablation corrects the hemodynamic abnormality in patients with CEAP clinical class 3-6 CVI due to superficial reflux. Vasc Endovascular Surg. 2006;40:125-130.
25. De Medeiros CA, Luccas GC. Comparison of endovenous treatment with an 810 nm laser versus conventional stripping of the great saphenous vein in patients with primary varicose veins. Dermatol Surg. 2005;31:1685-1694.
26. Mekako AI, Hatfield J, Bryce J, et al. A nonrandomised controlled trial of endovenous laser therapy and surgery in the treatment of varicose veins. Ann Vasc Surg. 2006;Jun 27:[Epub ahead of print].
27. Vuylsteke M, Van den Bussche D, Audenaert EA, Lissens P. Endovenous laser obliteration for the treatment of primary varicose veins. Phlebology. 2006;21:80-87. 28. Perkowski P, Ravi R, Gowda RC, et al. Endovenous laser ablation of the saphenous vein for treatment of venous insufficiency and varicose veins: early results from a large single-center experience. J Endovasc Ther. 2004;11:132-138.
29. Sharif MA, Soong CV, Lau LL, et al. Endovenous laser treatment for long saphenous vein incompetence. Br J Surg. 2006;93:831-835.
30. Proebstle TM, Krummenauer F, Gul D, Knop J. Nonocclusion and early reopening of the great saphenous vein after endovenous laser treatment is fluence dependent. Dermatol Surg. 2004;30:174-178.
31. Timperman PE, Sichlau M, Ryu RK. Greater energy delivery improves treatment success of endovenous laser treatment of incompetent saphenous veins. J Vasc Interv Radiol. 2004;15:1061-1063.
32. Proebstle TM, Sandhofer M, Kargl A, et al. Thermal damage of the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg. 2002;28:596-600.
33. Schmedt CG, Sroka R, Steckmeier S, et al. Investigation on radiofrequency and laser (980 nm) effects after endoluminal treatment of saphenous vein insufficiency in an ex-vivo model. Eur J Vasc Endovasc Surg. 2006;Jun 14;[Epub ahead of print].
34. Anderson RR. Endovenous laser: mechanism of action. Paper presented at the Annual Meeting of the American Academy of Dermatology; March 3-7, 2006; San Francisco, California, USA.
35. Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Annals of Surgical Oncology. 2003;10:491-497.
36. Mordon SR, Wassmer B and Zemmouri J. Mathematical modeling of endovenous laser treatment (ELT). BioMedical Engineering OnLine. 2006;5:26. Available at: http://www.biomedical-engineeringonline. com/content/5/1/26. Accessed July 19, 2006.
37. De-Fei Hong, Shu-You Peng, Song-Ying Li, Li-Min Tong. Experimental study of diodelaser induced thermocoagulation on hepatic tissue with scanner fiber tip. World J Gastroenterol. 2003;9:2350-2352.
38. Zimmet SE. Pain, bruising and short-term efficacy after endovenous laser treatment of the greater saphenous vein: the effect of operative technique and postoperative care. Paper presented at the 16th Annual Congress of the American College of Phlebology; Nov 7-10, 2002; Fort Lauderdale, Florida, USA.
39. Proebstle TM, Moehler T, Gul D, Herdemann S. Endovenous treatment of the great saphenous vein using a 1320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31:1678-1683.
40. Weiss RA. Comparison of endovenous radiofrequency versus 810 nm diode laser occlusion of large veins in an animal model. Dermatol Surg. 2002;28:56-61.
41. Biesman BS, Khan J. Laser incisional surgery. Clinics in Plastic Surg. 2000;27:213- 220.
42. Moritz AR, Henriques Jr EC. Studies of thermal injury II: the relative importance of time and surface temperature in the causation of cutaneous bums. Am J Pathol. 1947;23:695-720.
43. Li S, Chien S, Branemark P. Heat shockinduced necrosis and apoptosis in osteoblasts. J Orthop Res. 1999;17:891-899.
44. Jansen W, Haveman J. Histopathological changes in the skin and subcutaneous tissues of mouse legs after treatment with hyperthermia. Path Res Pract. 1990;186: 247-253.
45. Zimmet SE, Min RJ. Temperature changes in perivenous tissue during endovenous laser treatment in a swine model. J Vasc Interv Radiol. 2003;14:911-915.
46. Lahl W. Thermometric investigations of perivenous temperature during endovenous laser therapy of varicose veins. Paper presented at the 15th World Congress of the Union Internationale de Phlébologie; October 2-7, 2005; Rio de Janeiro, Brazil.
47. Beale RJ, Mavor AID, Gough MJ. Heat dissipation during endovenous laser treatment of varicose veins—is there a risk of nerve injury? Phlebology. 2006;21:32-35.
48. Chang CJ, Chua JJ. Endovenous laser photocoagulation (EVLP) for varicose veins. Lasers Surg Med. 2002;31:257-262.
49. Ricci S, Georgiev M, Jawien A, Zamboni P. Sciatic nerve varices. Eur J Vasc Endovasc Surg. 2005;29:83-87.
50 Ricci S. Ultrasound observation of the sciatic nerve and its branches at the popliteal fossa: always visible, never seen. Eur J Vasc Endovasc Surg. 2005;30:659-663. 51 Mozes G, Kalra M, Carmo M, Swenson L, Gloviczki P. Extension of saphenous thrombus into the femoral vein: a potential complication of new endovenous ablation techniques. J Vasc Surg. 2005;41:130-135.
52 Timperman, PE. Arteriovenous fistula after endovenous laser treatment of the short saphenous vein. J Vasc Interv Radiol. 2004;15:625-627.
53 Dunst KM, Huemer GM, Wayand W, Shamiyeh A. Diffuse phlegmonous phlebitis after endovenous laser treatment of the greater saphenous vein. J Vasc Surg. 2006;43:1056-1058.
54 Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003;38:207-214.
55 Merchant RF, DePalma RG, Kabnick LS. Endovascular obliteration of saphenous reflux: a multicenter study. J Vasc Surg. 2002;35:1190-1196.
56 Puggioni A, Kalra M, Carmo M, Mozes G, Gloviczki P. Endovenous laser therapy and radiofrequency ablation of the great saphenous vein: analysis of early efficacy and complications. J Vasc Surg. 2005;42; 488-493.
57 Forlee MV, Grouden M, Moore DJ, Shanik G. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg. 2006;43: 162-164.
