Effectiveness of treatment for pelvic congestion syndrome
Mark WHITELEY2;
Cees H. A. WITTENS1,3
Maastricht University Medical Centre+,
Cardiovascular Research Institute
Maastricht, Maastricht, The Netherlands
Department of Obstetrics and Gynaecology, Haga Teaching Hospital,
The Hague, The Netherlands.
2 The Whiteley Clinic and Faculty of Health and Biomedical Sciences,
University of Surrey. Guildford, UK
3 Department of Vascular Surgery,
University Hospital Aachen, Aachen, Germany
Abstract
Pelvic congestion syndrome accounts for approximately 16% to 31% of patients suffering from chronic pelvic pain, and it is the second most frequent cause of pelvic pain after endometriosis. It is a poorly understood disease, and various treatments have been suggested in the past. Hormonal treatment, which suppresses ovarian function, demonstrated varying results. Hysterectomy with salpingooophorectomy used to be the second option for treatment, though efficacy of this treatment is disputable. In the more recent past, endovascular techniques for abolishing pelvic vein incompetence have been introduced with varying success. Additionally, deep venous obstruction caused by left renal vein entrapment or iliac vein compression has been identified as an important component of pelvic pain. Percutaneous endovenous techniques seem to be the best alternative as the initial treatment option. Several studies have also suggested that psychosocial factors weigh heavily on treatment outcomes, so concurrent psychotherapy may be useful when treating these patients. Future research should focus on reproducibility of treatment procedures, and randomized controlled trials should determine whether treatment of pelvic venous obstruction or incompetence is useful in relieving chronic pelvic pain. Then, properly designed studies should identify the importance of treating obstruction before incompetence. Finally, the additive effect of psychotherapy should be investigated.
Introduction
Pelvic congestion syndrome (PCS) is a poorly understood syndrome that is characterized by a vague chronic pelvic pain that persists for at least 6 months.1-3 Pain is usually described as being dull or a feeling of heaviness on one side of the abdomen; however, more localized complaints have also been described.1,4,5 Complaints usually exacerbate around the menses or during and after coitus. As with venous incompetence, complaints are typically aggravated at the end of the day and during long periods of standing or walking, and can improve when assuming the supine position.1,5 The role of pregnancy is unclear in this syndrome, although complaints usually increase.1 Furthermore, PCS is associated with the presence of varicosities in the vulvovaginal, gluteal, peritoneal, and lower limb areas.1,3
The prevalence and incidence of PCS are unclear due to the broad differential diagnosis of pelvic pain, the unclear pathophysiology of PCS, and the difficulties in diagnosing PCS as the cause of pelvic complaints. PCS is the leading cause of chronic pelvic pain after endometriosis, and it is estimated to account for 16% to 31% of cases.5 Due to the poorly understood etiology, treatment of PCS can be a difficult venture. This paper will evaluate the effectiveness of possible treatment options for PCS.
Hormonal treatment
Since PCS usually occurs in premenopausal women and it is usually not found in postmenopausal women, it is hypothesized that hormones play a role in PCS.1,6 Therefore, hormone therapy that suppresses ovarian activity could be useful when treating PCS.7 Studies included in a recent systematic review diagnosed PCS using the criteria of Beard et al,8 while other causes of pelvic pain were excluded through laparoscopy and ultrasound. The Beard criteria focus on three venography findings, appointing a score of 1 to 3 to each finding: the maximum diameter of the ovarian vein (1-4 mm, 5-8 mm, or >8 mm), time of disappearance of contrast medium from end of injection (0 s, 20 s, or 40 s), and congestion of the ovarian plexus (normal, moderate, or extensive).9
Some studies have shown a beneficial effect of medroxyprogesterone (MPA) on a visual analog scale for pain.10-12 Reginald et al11 did not have a control group; however, after treating patients for 6 months with 30 mg MPA daily, patients with a venogram score reduction showed a 75% decrease in the visual analog scale score compared with only 29% in those without a venogram score change (P<0.01). Farquhar et al10 performed a well-executed randomized controlled trial with 4 groups: 4 months of 50 mg MPA, MPA + psychotherapy (6 sessions), placebo, and placebo + psychotherapy. At the end of treatment, 73% of the patients in the MPA groups showed a ≥50% reduction in visual analog scale scores compared with 33% in the two placebo groups (P<0.001). However, 9 months after cessation of treatment, the effect decreased for MPA and psychotherapy when administered alone, while the effect in the placebo group did not change. The combination of MPA and psychotherapy showed that 71% of patients had a ≥50% reduction in visual analog scale scores 9 months after cessation of therapy (P<0.05).
Although MPA appears to have a positive effect on pelvic pain in PCS, gonadotropin-releasing hormone (GnRH) agonists seem to have a superior effect. Soysal et al12 compared 30 mg MPA daily with a 3.6 mg goserelin acetate injection every month for 6 months. The final evaluation occurred 12 months after cessation of treatment, and the data demonstrated a 7.7±1.8 and 4.7±1.4 decrease in pelvic pain with goserelin acetate vs MPA, respectively (P=0.00001) on a modified version of the Biberoglu and Behrman13 pelvic symptom score (score varying from 0 to 12). However, no information on other treatments during the cessation period was given. A case series of 21 patients did not yield successful results with a combination of goserelin acetate, estradiol valerate, and MPA,14 yet in this small group, 2 patients underwent a hysterectomy or oophorectomy and 5 patients discontinued treatment during the study. Shokeir et al15 compared a subcutaneous etonogestrel insert with no treatment for 12 months, and the data showed a significant difference in visual analog scale scores between treatment and no treatment (7.7 before vs 2.4 after Implanon, 7.9 before vs 7.6 after with controls; P<0.05). However, patients were not blinded to the treatment.
Hysterectomy and salpingo-oophorectomy
Oophorectomy used to be considered for those who did not respond to hormone treatment. Oophorectomy was combined with hysterectomy to prevent withdrawal bleeding due to hormone replacement therapy.6 Beard et al6 reported a case series in which bilateral oophorectomy with hysterectomy, usually after failed hormone treatment, demonstrated a change from a median visual analog scale score of 10 to a median visual analog scale score of 0 at 1-year follow-up (P<0.001). A total of 24 patients (66.7%) demonstrated full relief from pain, 11 (30.6%) experienced significant improvement, and 1 (2.7%) showed only a slight improvement. Chung et al16 were not able to reproduce this effect in a randomized controlled trial comparing ovarian vein embolization with hysterectomy and unilateral salpingo-oophorectomy (USO) or bilateral salpingooophorectomy (BSO). Hysterectomy with USO did not show a significant reduction in visual analog scale scores (7.8 to 5.6; P>0.05), while hysterectomy with BSO showed a change in visual analog scale scores from 7.7 to 4.6 (unclear whether statistically significant). This randomized controlled trial had some limitations because the inclusion process was not transparent, patients were not blinded to the treatment and the randomization procedure was not described.
Elimination of ovarian vein and internal iliac vein incompetence
Congestion of blood in the pelvis can result from incompetent ovarian veins or internal iliac veins (Figure 1). Data on surgical correction of ovarian vein incompetence through ligation is scant.17,18 Focus had quickly been diverted to less invasive, percutaneous techniques.
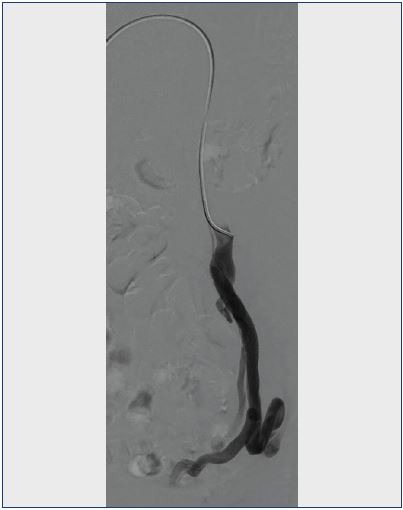 Figure 1. Venogram of an incompetent left ovarian vein with pelvic collateralization.
Figure 1. Venogram of an incompetent left ovarian vein with pelvic collateralization.
The study of Chung et al,16 which was discussed in the previous section, demonstrated a significant decrease in the visual analog scale scores for ovarian vein embolization (7.8 to 3.2; P<0.05) with better results than hysterectomy with USO or BSO. Furthermore, data suggest that a psychological effect is important in PCS, as fewer patients demonstrated improvement in complaints in the group who scored high on stress scoring questionnaires based on the revised social readjustment rating scale (40.2% for severe stress scores vs 56.4% for moderate and 61.5% for low). It should be noted that the group of patients with high-stress levels was small (7 severe vs 18 moderate and 27 low).
Other studies have not used a control group and these studies have been composed of prospective or retrospective case series.1,4,7,16,19-36 The definition of PCS varied in these studies and was not always clearly stated. While some studies used visual analog scale scores for outcome measurement, many studies only used general clinical improvement when reporting outcomes with complete relief of symptoms varying from 50% to 100%.20,21,27,29,31-33,35 Laborda et al28 described the largest population (n=202) with the longest follow-up (5 years). The mean visual analog scale score was 7.34±0.7 before the procedure compared with 0.78±1.2 at the 5-year follow-up (P<0.0001). However, only 179 patients reached the 5-year follow-up, and inclusion criteria for PCS were based on symptoms and venography results, as opposed to the criteria established by Beard et al.8 Also, patients were initially referred with lower limb varicosities, after which PCS symptoms were identified by a questionnaire. The left ovarian vein was coiled in all patients, while the right ovarian vein was coiled in 193 (95.5%), the left internal iliac vein in 184 (91.1%), and the right internal iliac vein in 149 (73.8%) patients. It should be noted that the average time for clinical improvement was 13.5±1.9 months in those with severe pain (8-10) compared with 9.1±1.1 months in those with moderate pain (5-7; P=0.001). Initial follow-up visits were planned for 1, 3, 6, and 12 months after treatment.
Nasser et al investigated patients who suffered from chronic pelvic pain for at least 6 months, in combination with tenderness at the ovarian point during a physical examination.30 If venography demonstrated an incompetent ovarian vein or internal iliac vein, coiling was performed. It was not stated how many patients were analyzed, although 113 women underwent embolization, of which 13 were lost to follow-up and therefore excluded from analysis. Embolization was done in 100% of left ovarian veins, 72% of right ovarian veins, 80% of left internal iliac veins, and 46% of right internal iliac veins. Mean visual analog scale scores decreased from 7.34±0.07 to 0.47±0.05 at the 1-year follow-up (P<0.001). All patients reported significant improvement with 53 patients having no pelvic pain and 47 patients showing a reduction in pain. Multivariate analysis showed that complaints of urinary urgency and lower limb symptoms yielded an odds ratio of 5.9 (95% CI, 1.5-23.4) and 5.3 (95% CI, 1.4-20.4), respectively, for the risk of incomplete resolution of symptoms. Furthermore, a larger diameter of the right ovarian vein was associated with a higher chance of complete clinical success.
Kim et al26 studied the effect of coil embolization with an injection of a foam sclerosant of the ovarian veins and internal iliac veins in 127 patients; 20 patients and 106 patients underwent unilateral or bilateral ovarian vein embolization, respectively, with 1 patient in whom the treatment was unknown. At 4 to 6 weeks after the initial procedure, venography was performed to test and treat for internal iliac vein incompetence (n=108 patients). Mean follow-up was 45±18 months, with complete follow-up for 97 patients. Visual analog scale scores were significantly reduced for overall pain (7.6±1.8 before and 2.9±2.8 after; P<0.000001), dyspareunia (3.3±3.7 before and 1.5±2.7 after; P<0.000001), and menstrual pain (4.9±4.2 before and 2.2±3.1 after; P<0.000001). A total of 80 patients demonstrated clinical improvement; 64 significant, 11 moderate, and 5 mild. A recurrence rate of 5% was observed. Patient selection for this study was not uniform, and it was initially based on clinical suspicion of PCS. The proportion of patients who were nulliparous was very high compared with other studies (63%), although no significant differences in treatment effects were observed between multiparous and nulliparous patients.
Other smaller studies have shown comparable results.19,22,24,25,34,36 Complications were infrequent and not severe.5 A retrospective analysis by van der Vleuten et al35 showed that 9 out of 21 patients underwent more than one embolization with Histoacryl. After the second embolization, 4 still showed no improvement and 1 worsened; 2 patients underwent a third and fourth embolization without any success. However, it should be noted that the results were obtained through a retrospective questionnaire at a mean of 18.1 months (range, 4-60 months) after the first intervention.
Treatment of the Nutcracker syndrome
Nutcracker syndrome is caused by a compression of the left renal vein due to entrapment between the superior mesenteric artery and the aorta (Figure 2).37 Hartung et al38 treated 5 patients with PCS, who also demonstrated left renal vein entrapment. All patients received a selfexpanding metallic stent (Wallstent, Boston Scientific- Schneider, Minneapolis, MN) in their left renal vein. The first patient experienced migration of a 20×60 mm stent into the retrohepatic inferior vena cava. The stent was snared and pulled back, although it adopted a transversal position in the inferior vena cava, just cephalad to the left renal vein, and was left in place. A new 16 mm stent was successfully placed and all subsequent patients immediately received a 16 mm stent. Patients received low-molecular-weight heparin for 15 days and clopidogrel for at least 6 months, and 2 patients received this treatment in combination with a vitamin K antagonist.
At 1 month after stenting, all patients experienced an improvement in their symptoms. Duplex examination showed a patent stent and relief of ovarian vein incompetence in the 3 patients who had not had a previous ovarian vein embolization. A total of 2 patients were asymptomatic at 4.2 and 26.5 months postintervention, 1 patient developed a different kind of pelvic pain at 15 months postintervention and underwent hysterectomy for endometriosis; no such signs were found during the laparoscopy before stenting, and 2 patients had symptom recurrence at 3 and 4 months postintervention.
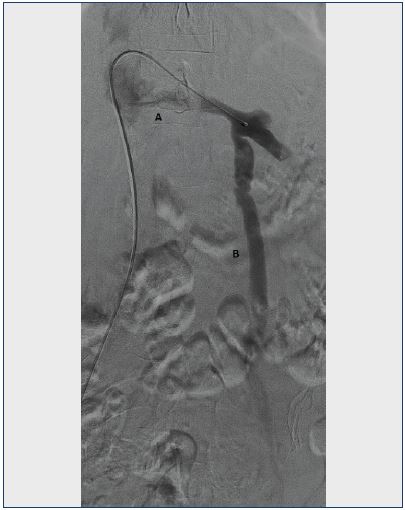 Figure 2. Venogram of the left renal vein.
Figure 2. Venogram of the left renal vein.
A. Lack of contrast filling in the renal vein due to the Nutcracker phenomenon.
B. Incompetent left ovarian vein as a collateral route for left renal outflow.
Duplex ultrasound and computed tomography showed minor stent migration to the right side of the inferior vena cava, which caused left renal vein compression to recur. No further complications were encountered. The indication for stenting was not uniform. It was based on symptomology, exclusion of other pathologies (all underwent laparoscopy), and confirmation of a Nutcracker syndrome on duplex ultrasound, computed tomography, and venography. Patients suffered from either PCS symptoms or flank pain, the latter occurring in 2 patients who initially reported with PCS complaints at 2 and 3 months after ovarian vein embolization.
A study by d’Archambeau et al23 described the presence of left renal vein compression in 40 out of 48 patients who underwent ovarian vein embolization; 12 patients showed extrinsic impression with retrograde filling of paralumbar veins and/or ovarian veins, 16 patients showed compression with filling of side branches including the ovarian vein and reflux toward the renal hilum, and 12 patients showed a total compression of the renal vein. Compared with other studies on ovarian vein embolization, a similar treatment effect was observed by only treating the ovarian vein incompetence and not treating the renal vein compression. However, both pre- and postprocedural visual analog scale scores were retrospectively obtained through a questionnaire at an unknown point after the procedure had already taken place. Moreover, no tests assessing kidney function were performed. Therefore, no information is available on kidney damage due to impaired renal outflow.
Nutcracker syndrome not only results in pelvic congestion, but can also cause symptoms like hematuria and proteinuria.39 Thus, other studies have also investigated treatment options; left renal vein transposition is the most commonly used technique and it has shown favorable results, with only two reported incidents of retroperitoneal hematoma, two of ileus, and one deep venous thrombosis.38,39
Treatment of iliac vein compression syndrome
Iliac vein compression can also lead to abdominal complaints (Figure 3), which was investigated by Daugherty et al.40 A total of 18 patients received a stent in their left common iliac vein, 1 was done in conjunction with coiling of the left ovarian vein, and 1 patient underwent stenting of the suprarenal inferior vena cava. At a median followup of 11 months (range, 1-59 months), 15 patients (79%) were asymptomatic and 4 (21%) reported substantial improvement in complaints. The median venous clinical severity score before the intervention was 7 (range, 0-10) and decreased to 3.5 (range, 0-9) after the intervention. This retrospective study included patients with both pelvic complaints that severely affected their quality of life and proven nonthrombotic venous outflow obstruction on duplex ultrasound and computed tomography. Patients who also had lower extremity complaints that were more important than the pelvic complaints were excluded. If intravascular ultrasound and venography confirmed a nonthrombotic lesion, a stent was placed. A total of 10 patients initially presented with lower leg complaints, but after anamnesis it became clear that pelvic complaints were more relevant than lower leg pain. A history of hysterectomy was present in 8 patients, which highlights the fact that hysterectomy does not necessarily lead to a reduction in PCS complaints.
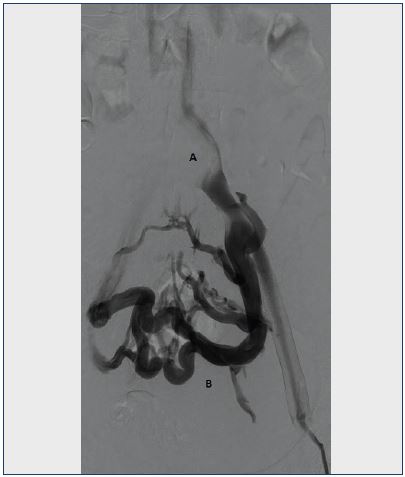 Figure 3. Venogram of the left venous iliac tract.
Figure 3. Venogram of the left venous iliac tract.
A. Lack of contrast filling in the left common iliac vein due to compression of the overlying iliac artery.
B. Extensive collateral formation in the pelvis.
Also, 7 ovarian veins were found to be incompetent, but only 1 ovarian vein needed treatment.
Stenting for iliac vein compression has been studied a lot for lower extremity complaints. In these studies, few complications were encountered and patency rates as high as 100% have been reported.41,42
Discussion
Several treatment options exist for PCS. Medical treatment with MPA or GnRH agonists can be considered, although a considerable amount of patients do not experience adequate relief from their complaints and side effects are frequent and uncomfortable.5,7 In the past, the next step was hysterectomy with salpingo-oophorectomy; however, efficacy of this procedure is unclear as Beard et al6 reported good clinical results, while Chung et al16 did not. The high prevalence of hysterectomies in patients with PCS in the study by Daugherty et al40 could be an indication that such a surgical procedure should be avoided if possible, especially considering the invasiveness of the procedure compared with endovascular approaches.
Chung et al16 did show a significant effect of ovarian vein embolization on pelvic pain reduction. Although this was the only randomized controlled trial, several large studies have shown a positive effect by ovarian and/or internal iliac vein embolization on pelvic pain, dyspareunia, and menstrual pain.26,28,30 Patience may be required as the duration before improvement can be 9.3 to 13.5 months, with earlier improvement in those with less severe complaints.28 Furthermore, concurrent lower limb complaints and urinary urgency have been identified as independent risk factors for incomplete treatment success.30
Reports on unresponsiveness to PCS treatment vary; 6% to 53% of patients show no improvement.5 Although the overall heterogeneity and methodological imperfections can partly account for this variation, it is likely that a psychosocial component should not be overlooked. Patients who scored high on the revised social readjustment rating scale seem to respond less to embolization treatment,16 whereas patients with psychotherapy appear to have a longer lasting effect from the hormone treatment.10 Therefore, unresponsiveness to treatment should not necessarily lead to a reintervention, especially since odds of success appear to be limited.35 Also, not all people who show signs of venous congestion in the pelvic area, present with symptoms of pelvic pain. Several studies have shown that 5% to 63% of patients in a population without pelvic pain suffer from some form of pelvic congestion.43-46
No recommendations can be made for a method of embolization. Coiling, foam, and glue seem to yield similar outcomes, although no proper comparisons have been performed. Thus, no clear conclusions can be drawn. It is also difficult to compare the individual effect of either ovarian vein or internal iliac vein embolization since studies have used both techniques without subanalyses. Evidence suggests that when both ovarian and internal iliac vein incompetence are present, treating ovarian vein incompetence alone does not lead to adequate relief of symptoms.19
The possible presence of deep vein obstruction in PCS should not be forgotten. Compression of the left renal vein or common iliac vein can lead to pelvic congestion, which can be treated by venous stenting.38,40 Stenting of the left renal vein is not suggested as the first choice of treatment because data on this type of stenting is scarce and stent migration is frequent. However, no real conclusions should be drawn from experience in only 5 patients with one particular type of stent. New dedicated stents might be far more appropriate for left renal vein stenting, which could decrease the risk of migration and make stenting a first-line treatment. Ovarian vein embolization might be effective in the presence of renal vein entrapment,23 yet this is not the preferred hemodynamic option. No data exist about the effect on kidney function, which might be impaired since renal vein entrapment pertains to an outflow obstruction of a highly perfused organ. Before performing ovarian vein embolization, it is probably essential to evaluate whether a Nutcracker or iliac vein compression is present, because occluding the ovarian vein is, in fact, occluding the outflow of the renal vein or occluding the collateral circulation developed due to the iliac vein obstruction. It seems logical to treat obstruction first, if simultaneously present, and treat persistent symptomatic incompetence second, as collateral function has been suggested to be important.47
In conclusion, pelvic congestion syndrome is a poorly understood disease with several treatment options. Percutaneous endovenous techniques seem to be the best choice as an initial treatment option. However, psychosocial factors appear to weigh heavily on treatment outcomes. Future research should focus on a number of aspects. First, reproducibility of treatment procedures and randomized controlled trials should determine whether treatment of pelvic venous obstruction or incompetence is useful in relieving chronic pelvic pain. Second, properly designed studies should identify the importance of treating obstruction before incompetence. Finally, the additive effect of psychotherapy should be investigated.
REFERENCES
1. Borghi C, Dell’Atti L. Pelvic congestion syndrome: the current state of the literature. Arch Gynecol Obstet. 2016;293(2):291-301.
2. Williams RE, Hartmann KE, Steege JF. Documenting the current definitions of chronic pelvic pain: implications for research. Obstet Gynecol. 2004;103(4):686-691.
3. Hobbs JT. The pelvic congestion syndrome. Br J Hosp Med. 1990;43(3):200-206.
4. Hansrani V, Abbas A, Bhandari S, Caress AL, Seif M, McCollum CN. Transvenous occlusion of incompetent pelvic veins for chronic pelvic pain in women: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2015;185:156- 163.
5. Meissner MH, Gibson K. Clinical outcome after treatment of pelvic congestion syndrome: sense and nonsense. Phlebology. 2015;30(suppl 1):73-80.
6. Beard RW, Kennedy RG, Gangar KF, et al. Bilateral oophorectomy and hysterectomy in the treatment of intractable pelvic pain associated with pelvic congestion. Br J Obstet Gynaecol. 1991;98(10):988-992.
7. Tu FF, Hahn D, Steege JF. Pelvic congestion syndrome-associated pelvic pain: a systematic review of diagnosis and management. Obstet Gynecol Surv. 2010;65(5):332-340.
8. Beard RW, Highman JH, Pearce S, Reginald PW. Diagnosis of pelvic varicosities in women with chronic pelvic pain. Lancet. 1984;2(8409):946-949.
9. Kauppila A. Uterine phlebography with venous compression. A clinical and roentgenological study. Acta Obstet Gynecol Scand Suppl. 1970;3(suppl 3):1-66.
10. Farquhar CM, Rogers V, Franks S, Pearce S, Wadsworth J, Beard RW. A randomized controlled trial of medroxyprogesterone acetate and psychotherapy for the treatment of pelvic congestion. Br J Obstet Gynaecol. 1989;96(10):1153-1162.
11. Reginald PW, Adams J, Franks S, Wadsworth J, Beard RW. Medroxyprogesterone acetate in the treatment of pelvic pain due to venous congestion. Br J Obstet Gynaecol. 1989;96(10):1148-1152.
12. Soysal ME, Soysal S, Vicdan K, Ozer S. A randomized controlled trial of goserelin and medroxyprogesterone acetate in the treatment of pelvic congestion. Hum Reprod. 2001;16(5):931-939.
13. Biberoglu KO, Behrman SJ. Dosage aspects of danazol therapy in endometriosis: short-term and long-term effectiveness. Am J Obstet Gynecol. 1981;139(6):645-654.
14. Gangar KF, Stones RW, Saunders D, et al. An alternative to hysterectomy? GnRH analogue combined with hormone replacement therapy. Br J Obstet Gynaecol. 1993;100(4):360- 364.
15. Shokeir T, Amr M, Abdelshaheed M. The efficacy of Implanon for the treatment of chronic pelvic pain associated with pelvic congestion: 1-year randomized controlled pilot study. Arch Gynecol Obstet. 2009;280(3):437-443.
16. Chung MH, Huh CY. Comparison of treatments for pelvic congestion syndrome. Tohoku J Exp Med. 2003;201(3):131-138.
17. Gargiulo T, Mais V, Brokaj L, Cossu E, Melis GB. Bilateral laparoscopic transperitoneal ligation of ovarian veins for treatment of pelvic congestion syndrome. J Am Assoc Gynecol Laparosc. 2003;10(4):501-504.
18. Rundqvist E, Sandholm LE, Larsson G. Treatment of pelvic varicosities causing lower abdominal pain with extraperitoneal resection of the left ovarian vein. Ann Chir Gynaecol. 1984;73(6):339-341.
19. Asciutto G, Asciutto KC, Mumme A, Geier B. Pelvic venous incompetence: reflux patterns and treatment results. Eur J Vasc Endovasc Surg. 2009;38(3):381- 386.
20. Bachar GN, Belenky A, Greif F, et al. Initial experience with ovarian vein embolization for the treatment of chronic pelvic pain syndrome. Isr Med Assoc J. 2005;(12):843-846.
21. Capasso P, Simons C, Trotteur G, Dondelinger RF, Henroteaux D, Gaspard U. Treatment of symptomatic pelvic varices by ovarian vein embolization. Cardiovasc Intervent Radiol. 1997;20(2):107-111.
22. Creton D, Hennequin L, Kohler F, Allaert FA. Embolisation of symptomatic pelvic veins in women presenting with nonsaphenous varicose veins of pelvic origin – three-year follow-up. Eur J Vasc Endovasc Surg. 2007;34(1):112-117.
23. d’Archambeau O, Maes M, De Schepper AM. The pelvic congestion syndrome: role of the “nutcracker phenomenon” and results of endovascular treatment. JBR-BTR. 2004;87(1):1-8.
24. Gandini R, Chiocchi M, Konda D, Pampana E, Fabiano S, Simonetti G. Transcatheter foam sclerotherapy of symptomatic female varicocele with sodium-tetradecyl-sulfate foam. Cardiovasc Intervent Radiol. 2008;31(4):778-784.
25. Hocquelet A, Le Bras Y, Balian E, et al. Evaluation of the efficacy of endovascular treatment of pelvic congestion syndrome. Diagn Interv Imaging. 2014;95(3):301-306.
26. Kim HS, Malhotra AD, Rowe PC, Lee JM, Venbrux AC. Embolotherapy for pelvic congestion syndrome: long-term results. J Vasc Interv Radiol. 2006;17(2 Pt 1):289-297.
27. Kwon SH, Oh JH, Ko KR, Park HC, Huh JY. Transcatheter ovarian vein embolization using coils for the treatment of pelvic congestion syndrome. Cardiovasc Intervent Radiol. 2007;30(4):655-661.
28. Laborda A, Medrano J, de Blas I, Urtiaga I, Carnevale FC, de Gregorio MA. Endovascular treatment of pelvic congestion syndrome: visual analog scale (VAS) long-term follow-up clinical evaluation in 202 patients. Cardiovasc Intervent Radiol. 2013;36(4):1006-1014.
29. Maleux G, Stockx L, Wilms G, Marchal G. Ovarian vein embolization for the treatment of pelvic congestion syndrome: long-term technical and clinical results. J Vasc Interv Radiol. 2000;11(7):859-864.
30. Nasser F, Cavalcante RN, Affonso BB, Messina ML, Carnevale FC, de Gregorio MA. Safety, efficacy, and prognostic factors in endovascular treatment of pelvic congestion syndrome. Int J Gynaecol Obstet. 2014;125(1):65-68.
31. Pieri S, Agresti P, Morucci M, de’ Medici L. Percutaneous treatment of pelvic congestion syndrome. Radiol Med. 2003;105(1-2):76-82.
32. Tarazov PG, Prozorovskij KV, Ryzhkov VK. Pelvic pain syndrome caused by ovarian varices. Treatment by transcatheter embolization. Acta Radiol. 1997;38(6):1023-1025.
33. Tinelli A, Prudenzano R, Torsello M, et al. Suprapubic percutaneous sclero-embolization of symptomatic female pelvic varicocele under local anesthesia. Eur Rev Med Pharmacol Sci. 2012;16(1):111-1117.
34. Tropeano G, Di Stasi C, Amoroso S, Cina A, Scambia G. Ovarian vein incompetence: a potential cause of chronic pelvic pain in women. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):215-221.
35. van der Vleuten CJ, van Kempen JA, Schultze-Kool LJ. Embolization to treat pelvic congestion syndrome and vulval varicose veins. Int J Gynaecol Obstet. 2012;118(3):227-230.
36. Venbrux AC, Chang AH, Kim HS, et al. Pelvic congestion syndrome (pelvic venous incompetence): impact of ovarian and internal iliac vein embolotherapy on menstrual cycle and chronic pelvic pain. J Vasc Interv Radiol. 2002;13(2 Pt 1):171-178.
37. El-Sadr AR, Mina E. Anatomical and surgical aspects in the operative management of varicocele. Urol Cutaneous Rev. 1950;54(5):257-262.
38. Hartung O, Grisoli D, Boufi M, et al. Endovascular stenting in the treatment of pelvic vein congestion caused by nutcracker syndrome: lessons learned from the first five cases. J Vasc Surg. 2005;42(2):275-280.
39. Hohenfellner M, D’Elia G, Hampel C, Dahms S, Thüroff JW. Transposition of the left renal vein for treatment of the nutcracker phenomenon: long-term follow-up. Urology. 2002;59(3):354- 357.
40. Daugherty SF, Gillespie DL. Venous angioplasty and stenting improve pelvic congestion syndrome caused by venous outflow obstruction. J Vasc Surg Venous Lymphat Disord. 2015;3(3):283-289.
41. Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(5):979- 990.
42. de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short-term clinical experience with a dedicated venous nitinol stent: initial results with the sinus-venous stent. Eur J Vasc Endovasc Surg. 2015;50(4):518- 526.
43. Ball E, Khan KS, Meads C. Does pelvic venous congestion syndrome exist and can it be treated? Acta Obstet Gynecol Scand. 2012;91(5):525-528.
44. Nascimento AB, Mitchell DG, Holland G. Ovarian veins: magnetic resonance imaging findings in an asymptomatic population. J Magn Reson Imaging. 2002;15(5):551-556.
45. Rozenblit AM, Ricci ZJ, Tuvia J, Amis ES Jr. Incompetent and dilated ovarian veins: a common CT finding in asymptomatic parous women. AJR Am J Roentgenol. 2001;176(1):119-122.
46. Belenky A, Bartal G, Atar E, Cohen M, Bachar GN. Ovarian varices in healthy female kidney donors: incidence, morbidity, and clinical outcome. AJR Am J Roentgenol. 2002;179(3):625-627.
47. Kurstjens RLM, de Wolf MAF, van Laanen JHH, de Haan MW, Wittens CHA, de Graaf R. Hemodynamic significance of collateral blood flow in chronic venous obstruction. Phlebology. 2015;30(suppl 1):27-34.

