Cyanoacrylate closure in the treatment of varicose veins – what is the evidence?
DBVLM
Arizona, USA
Abstract
Introduction
Cyanoacrylate ablation for incompetent saphenous veins is a recent addition to the armamentarium of venous surgeons. It does not require the instillation of tumescent anesthesia during the procedure, thus reducing patient discomfort, and neither are compression hose necessary after treatment.
Early cyanoacrylate ablation trials
VenaSeal™ (Medtronic, Minnesota, USA) was the first reported use of a formulation of cyanoacrylate used in the ablation of incompetent saphenous veins. Clinical series and randomized controlled clinical trials demonstrated the safety and efficacy of this ablation method. The VeClose trial compared VenaSeal™; to the radiofrequency ablation (RFA) method and showed clear noninferiority of safety and efficacy compared with RFA.
Subsequent VenaSeal™ trials
The WAVES trial demonstrated efficacy of VenaSeal™ in large great saphenous veins without the use of compression hose. And in a 60-month extension study of patients from the VeClose trial, long-term occlusion success and freedom from adverse events has been confirmed.
Other cyanoacrylate-formulation studies
Among alternative cyanoacrylate formulations first reported was N-butyl cyanoacrylate adhesive (NBCA), used successfully in saphenous vein ablations in Turkey. Several trial reports have been published comparing NBCA with thermal ablation techniques, showing equal or better efficacy and safety for NBCA ablation.
Conclusion
The safety and efficacy of differing formulations of cyanoacrylate for ablation of incompetent saphenous veins have been demonstrated through many clinical trials internationally.
Introduction
The incidence of chronic venous disease (CVD) in the population overall is 18.2% and increases with advancing age.1 The progression of CVD as evidenced by advancing clinical classification significantly impacts a patient’s quality of life (QOL).2,3
There has been a dramatic evolution over the past 3 decades in the management of CVD in general and saphenous insufficiency in particular with minimally invasive endovenous techniques replacing conventional surgical therapy.4
The safety and efficacy of endovenous therapies, including radiofrequency ablation (RFA), ultrasound-guided foam sclerotherapy, and endovenous laser ablation (EVLA), have been reported in numerous clinical trials.5-10
In the recently published clinical guidelines from the European Society of Vascular Surgery, for patients requiring intervention for superficial truncal venous incompetence, endovenous thermal ablation is recommended at a class I level A; endovenous ultrasound-guided foam sclerotherapy of smaller superficial truncal vein incompetence at a IIb Level B.11
Some concern has been raised in the literature about neurosensory adverse events in association with ultra sound guided foam sclerotherapy. Initially, a report was published in the Journal of Vascular Surgery in 2006 by Forlee and colleagues12 regarding a patient who developed a stroke following foam sclerotherapy attributed to gas bubbles passing through a previously undiagnosed right-to-left intracardiac shunt (18-mm patent foramen ovale). An immediate carotid duplex scan demonstrated moving echogenic particles consistent with gas bubbles, although magnetic resonance imaging (MRI) of the brain was normal. The patient recovered most of his neurological deficits, but the report was cause for great concern in the international phlebologic community. Because of this and a number of other reports of neurosensory adverse events, there remains some concern about the intravenous injection of foam in the treatment of what is almost exclusively a nonlethal condition and has stimulated investigations into alternative ablation methods.
RFA and EVLA require the use of tumescent anesthesia (TA) and post-procedural compression stockings, both of which often produce discomfort during and after the procedure.
To address the discomfort associated with TA, newer, nonthermal nontumescent therapies (NTNT) for the treatment of saphenous insufficiency have been introduced. One relatively recent NTNT technique is the use of cyanoacrylate (CA) adhesive to produce occlusion and eventual fibrosis of the saphenous trunk. The first CA developed for this purpose was VenaSeal™ (Medtronic, Minnesota, USA), which has been most commonly used in clinical trials originating in the USA,13 western Europe,14 and much of Asia (see attached video demonstration of procedure using VenaSeal™).15
The first-in-human trial reported by Almeida16 demonstrated occlusion of the GSV in 92% of the patient cohort at 1 year, along with significant reduction (improvement) in the Venous Clinical Severity Score (VCSS). Soon thereafter, in a single-arm, multicenter, cohort study, the European Sapheon™ Closure System Observational Prospective study (eSCOPEstudy), Proebstle et al published an occlusion rate at 12 months of 92.1%.17 Subsequently, the randomized controlled VeClose trial was published in which the VenaSeal™ adhesive was compared with RFA in a noninferiority trial.12 This was a prospective randomized controlled trial into which 242 patients were enrolled, with the first 20 patients used as roll-in to assure investigators were familiar with the procedure.18 All investigators were experienced endovenous surgeons. Patients aged 21 to 70 years in Clinical, Etiologic, Anatomic, and Pathophysiologic (CEAP) class C2-C4b with symptomatic GSV incompetence and a reflux time of ≥0.5 seconds assessed in the standing position with duplex ultrasound were enrolled. Patients with significant reflux of the small saphenous vein or anterior accessory GSV, who had previous treatment for venous disease in the target limb, symptomatic peripheral arterial disease, history of deep venous thrombosis or pulmonary embolism, or aneurysm of the target GSV with >12-mm diameter were excluded from the trial.
Patients were randomized into 2 groups: those treated with RFA and those treated with CA (VenaSeal™). Treatment was confined to the GSV only without adjunctive treatment for 3 months following the index procedure. The primary end point of occlusion of the GSV at 3 months without any patent segment >5 cm was achieved in 99% of the CA group and 96% of the RFA group, thus demonstrating noninferiority of CA vs RFA. Nearly equal improvement in the secondary end points of VCSS score QOL instruments was reported; no deep venous thromboses (DVTs) were identified in either group; and there was no significant difference in side effects or complication, including phlebitis. At 1 year, nearly identical occlusion rates were seen in the CA and RFA group (97.2% for CA vs 97% for RFA).10 At that time point, disease-specific and generic QOL improvement was also similar in the 2 groups and the inflammation seen early on in patients from both groups had subsided on its own or with the addition of a brief course of over-the-counter anti-inflammatory medication. In the subsequent 24-month19 and 36-month20 follow-up of the VeClose trial, the occlusion rates were identical in both the CA and RFA groups, and parallel improvements in the VCSS and QOL scores were found, demonstrating durable noninferiority of CA closure (CAC) compared with RFA. There were 5 adverse events in the CA group between 24 and 36 months, 2 of which were related to the procedure. During the same time period, there were 4 serious adverse events in the CA group, none of which were related to the procedure (liver cancer, breast cancer, cervical pain, and suicide attempt).
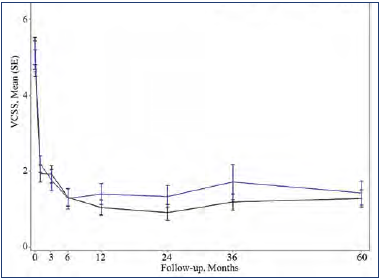
Figure 1. Mean Venous Clinical Severity Score (VCSS) during a 60-month follow-up period of eligible patients from the VeClose trial. Signs/symptoms associated with venous reflux disease (assessed by investigator via VCSS score, with scores ranging from 0 [no venous disease] to 30 [severe venous disease]) improved over time and was maintained through 60 months. Reduction from baseline: VenaSeal™ CS, 75%; radiofrequency ablation, 72%. After reference 21: Morrison et al. J Vasc Surg Venous Lymphat Dis. 2020;8(6):978-989. © 2020 The Authors. Published by Elsevier Inc. on behalf of the Society for Vascular Surgery.
Long-term follow-up studies are required to establish the durability of the treatment in terms of efficacy and safety. The first to be published was a 5-year extension study of patients from the VeClose trial aimed to assess the long-term efficacy and safety of CA and RFA in patients with incompetent GSV.21 The study included a 36-to-60-month interval evaluation of eligible patients from the VeClose trial for occlusion rates, 60-month CEAP classification, VCSS and QOL scores, patient satisfaction with treatment, need for adjunctive treatment, and adverse events. At month 60, VCSS score improvement was maintained (Figure 1), and complete occlusion of the GSV was reported in 94.6% of patients who had undergone VenaSeal™ ablation (CAC method) and 100% of patients in the RFA group (P=0.292) (Table I). No C0 or C1 patients were enrolled at the outset of the study, but interestingly, by the end of the extension study, 29/47 patients having undergone CA ablation were then classified as C0 or C1 (Figure 2). Presumably, if the investigators had been more successful with patient recruitment, even more C0 and C1 patients would have been identified. QOL improvements were maintained over the long term, and the need for adjunctive treatment was minimal during this 36-to-60-month time interval (5 had sclerotherapy, no patient had phlebectomy). There were no adverse events from 36 to 60 months, including pulmonary embolisms (PEs), DVTs, and of note, no long-term inflammatory or hypersensitivity reactions. And finally, 100% of the patients in the study were either very or somewhat satisfied with their treatment. Table II shows overall conclusions of the study.
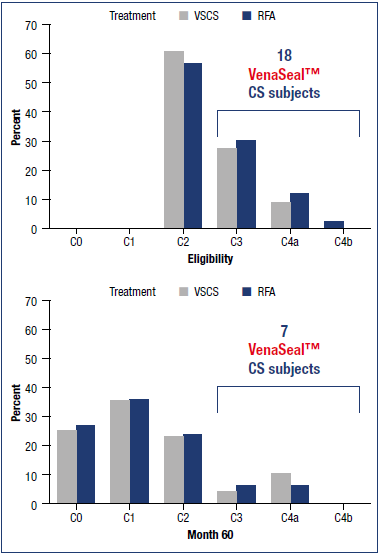
Figure 2. Results from the VeClose trial showing secondary end point of changes in C clinical classification (Clinical, Etiologic, Anatomic, and Pathophysiologic [CEAP] classification system]: C classification of subjects at eligibility and at 60 months with cyanoacrylate closure (VenaSeal™ CS) and radiofrequency ablation. Changes in C clinical classification were observed in all subjects. Abbreviations: RFA, radiofrequence ablation; VSCS, VenaSeal™ closure system. Based on reference 21: Morrison et al. J Vasc Surg Venous Lymphat Dis. 2020;8(6):978-989.
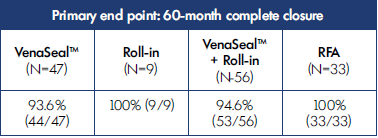
Table I. 5-year results from the VeClose trial showing primary end point of complete closure with cyanoacrylate closure (VenaSeal™ closure system) or radiofrequency ablation (RFA). 53 Subjects treated with VenaSeal™ closure system maintained a closure rate of 94.6% at 60 months. Based on reference 21: Morrison et al. J Vasc Surg Venous Lymphat Dis. 2020;8(6):978-989.
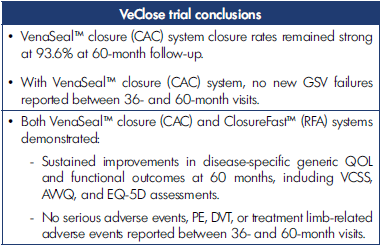
Table II. Overall conclusions from VeClose trial with cyanoacrylate closure (VenaSeal™ closure system) or radiofrequency ablation (ClosureFast™). Abbreviations: AVVQ, Aberdeen Varicose Vein Questionnaire; DVT, deep venous thrombosis; EQ-5D, EuroQol-5 Dimension; GSV, great saphenous vein; PE, pulmonary embolism; VCSS, Venous Clinical Severity Score. Based on reference 21: Morrison et al. J Vasc Surg Venous Lymphat Dis. 2020;8(6):978-989.
An important limitation of the 60-month extension study was that 89 of the original 242 patients agreed to the 60-month evaluation. Patients were enrolled in the VeClose trial with the understanding that the follow-up period would be 36 months. It proved difficult to find and convince subjects to participate in the 60-month follow-up in-person visit. However, patients in this study group were evenly divided between those who had RFA and those who had CAC.
Other VenaSeal™ trials
A 1-year GSV occlusion rate of 78.5% was reported by Chan and colleagues,22 with improvement of VCSS and generic and disease-specific QOL scores using VenaSeal™. Larger vein diameter (≥8 mm) was predictive of incomplete occlusion.
Hwang and colleagues23 expounded on the concept first reported by Gibson24 of the diminished need for adjunctive therapy for varicose veins following adhesive ablation of the GSV. In Gibson’s report, successful occlusion was achieved in all patients despite the absence of compression hose and adjunctive treatment. In the Hwang report, complete occlusion of all treated GSVs at 3 months was seen. Even more importantly, regression of varicose tributaries occurred in 71.7% and complete or >50% regression occurred in 90%.
In many CA trials, the use of compression hose post procedure has not been required. In the VeClose trial,12 because compression hose is standard procedure following RFA, and because this was a head-to-head comparison, compression hose were used in all patients, including those undergoing CA ablation. However, in the WAVES trial (Lake Washington Vascular VenaSeal™ Post-Market Evaluation)24 reported by Gibson and colleagues, compression hose were not used, even for patients with GSVs >10 mm in diameter, with no difference in occlusion rates.
In an early report from Lane and colleagues,25 there appeared to be a suggestion that treatment with CA in a patient on anticoagulation may not lead to successful vein ablation. That has not been the experience of this investigator, and to my knowledge no other similar reports have appeared in the literature.
In another retrospective review of 335 patients treated with VenaSeal™ compared with RFA, Yang and colleagues reported 100% successful ablation at 2 months.26
To improve occlusion rates in patients with GSVs >8 mm in diameter, Chan et al27 have applied an extra 0.09 mL of VenaSeal™ at the most proximal saphenous treatment site. Whereas occlusion rates significantly improved compared with the standard volume of adhesive, the occlusion rate was still not as high as in saphenous veins <8 mm in diameter. The study also determined that the extra drop of adhesive did not increase the rate of adhesive extrusion through the saphenofemoral junction. Interim 1-year results reported by Tang et al28 demonstrated a 12-month occlusion rate of 97.9%. At 3 months, revised VCSS and QOL scores were significantly improved in all patients, but between 3-month and 12-month follow-up there was no further improvement, nor were there any adverse events.
Park has published a case report of a patient with a 2.84-cm diameter GSV undergoing successful VenaSeal™ ablation by depositing additional adhesive in the dilated areas of the GSV.29
Vicente-Jimenez and colleagues in two hospitals in Spain retrospectively studied 233 patients who had undergone surgical stripping (SS), RFA, or CA adhesive ablation for incompetent saphenous veins.30 The clinical outcomes were measured by quality-adjusted life years (QALYs), complications, and reintervention, with a cost-effectiveness analysis comparing the 3 ablation methods. Clinical outcomes were essentially the same for RFA and CA, but the complication rate for SS was roughly 4 times that of RFA or CA. Cost-effectiveness analysis revealed that whereas health care costs only favored SS, CA was the most cost effective when direct health care costs were added to the cost of workdays lost.
Cyanoacrylate (VenaSeal™) perforator ablation
The feasibility of treating incompetent perforator veins with CA was studied in 33 incompetent perforator veins by Toonder et al.31 Occlusion rate at 3 months was 76%. In a subsequent retrospective review of 367 patients, Gibson32 treated 56 incompetent perforator veins in combination with CA ablation of superficial truncal veins. An occlusion rate of 85% at 1 month was demonstrated. And in a more recent publication, Mordhorst et al33 report 83 perforator veins were treated with VenaSeal™ with 86.5% occluded at 6 weeks.
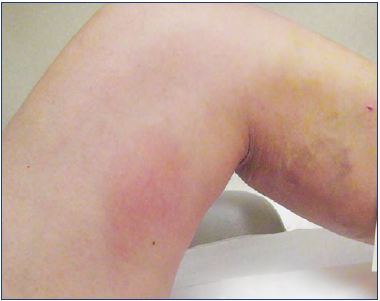
Figure 3. Inflammation in the thigh after cyanoacrylate ablation. Photo provided courtesy of Nick Morrison.
In a retrospective review of CEAP 6 venous leg ulcer patients undergoing VenaSeal™ ablation (CAC) or ClosureFast™ thermal ablation of saphenous veins, Kiguchi and colleagues34 reported a less frequent need to treat perforator veins following VenaSeal™ ablation than after ClosureFastR thermal ablation. It is theorized by the authors that treatment of a longer saphenous vein segment made possible with VenaSeal™ without the risk of nerve injury attendant to thermal ablation techniques is the reason for less-frequent subsequent perforator treatment in venous leg ulcer patients.
International cyanoacrylate alternative formulation trials
More recently, a number of clinical trials from various countries have been published also documenting the safety and efficacy of other formulations of CA adhesives.
In a randomized controlled trial comparing N-butyl cyanoacrylate adhesive (NBCA; VariClose System, Biolas, FG Grup, Turkey) with RFA and EVLA published by Eroglu and Yasim,35 at 2 years, all 3 groups experienced similar occlusion rates (NBCA 92.6%, RFA 90.9%, and EVLA 91.5%, P=0.89). with less periprocedural pain, faster return to work, and more improvement in VCSS scores for the group treated with NBCA.
In a prospective comparative study of CA (“Turkish Glue Kit”) vs EVLA for GSV treatment involving 208 CA procedures, Calik et al reported the 12-month occlusion rate for the CA patients was 96.6%.36 Similar improvements were seen in VCSS scores and values from the Chronic Venous Insufficiency Quality of Life Questionnaire (CIVIQ version 2) between the CA group and the EVLA group. Procedural pain was less, and induration, ecchymosis, and rate of paresthesia were all significantly less in the CA group. And in a retrospective analysis from Daylan and colleagues of 246 patients who had undergone CA (VenaBlock, Ankara, Turkey), at 5 years, the occlusion rate was 91.1%.37 The VCSS and Aberdeen Varicose Vein Questionnaire (AVVQ) scores significantly improved.
A report from India by Premnath and colleagues38 describes 124 patients undergoing saphenous and perforator ablation (269 in the group) using “commonly available n-butyl [CA] glue (which is used as topical skin adhesive or for endovascular embolization of arteriovenous malformations and vascular tumors)” (EndocrylR, Samarth Pharma Pvt Ltd, India).
Complications associated with adhesive ablation
The viscosity of the different formulations of CA and their associated rates of complications has been the subject of discussion in medical conferences.
VenaSeal™ is very viscous and polymerizes in less than 2 minutes. Adhesive manufactured in Turkey and India (Variclose®, Endocryl®), on the other hand have essentially the same viscosity of water but is said to polymerize in a matter of seconds.35,37
The importance of this difference is that embolization of adhesive can theoretically more readily be seen with adhesive of lower viscosity, thus increasing the risk of DVT. Premnath et al reported 96.5% occlusion rates at 1 year with all venous ulcerations healed but with 3/145 legs treated showing DVT, suggesting easier migration of the less viscous adhesive (Endocryl®, Samarth Pharmathan, India) than VenaSeal™.38 However, Cho et al reported a thrombus extension rate of 3.5% after VenaSeal™ ablation of GSVs.39
In a systematic review of 17 studies40 regarding CA ablation for truncal superficial veins of 1981 patients, among which up to 2-year occlusion rates were 93.7% and inflammatory reactions were seen less frequently after NBCA ablation than after RFA or EVLA, VCSS and QOL scores improved after the adhesive ablations. No differentiation was made by the authors between CA adhesive of different formulations. Complications such as bruising, phlebitis, and pain were seen less frequently in the NBCA group than in thermal ablation groups.
Chan, et al41 have recently reported a review of several short and mid-term clinical trials using VenaSeal™ in the Asian population, with 1-year occlusion rates of 90% in patients with GSV diameters <6.6 mm, low rates of DVT, and rates of inflammatory reaction up to 25.4%. A diameter >6.6 mm was a risk factor for recanalization of the target vein.
In the Cho retrospective review39 of 191 patients having had VenaSeal™ ablation of saphenous veins, extrusion of adhesive through the saphenofemoral junction or saphenopopliteal junction was seen in 5.8%, all limited to <50% of the common femoral vein lumen. Anticoagulation was not deemed necessary, and no further complications were identified. Diffuse inflammation in the thigh is commonly seen after CA ablation of the GSV (Figure 3), and readily responds to anti-inflammatory and antipruritic medications.20 Park et al described it as a “phlebitis-like abnormal reaction (PLAR)” with quite liberal criteria and thus occurring in 25.4% in their series of 271 veins treated.42
It has been the experience of this author that in the presence of an inflammatory reaction, compression hose routinely provides comfort to the patient and that an inflammatory reaction does not seem to affect occlusion of the saphenous vein as shown in a case report from Fiengo and colleagues.43
The hypersensitivity reaction seen in some patients after CA adhesive ablation is an erythematous effect generally near the venous treatment, with symptoms ranging from mild pruritis and/or erythema requiring no treatment for resolution to rare recurrent severe inflammation and pruritis. In an excellent discussion from a combined retrospective/ prospective review of 286 patients from Gibson et al, in which 379 veins were treated with VenaSeal™,44 hypersensitivity was seen in 6.3% of patients and were subdivided into mild presentations (4.2%) requiring either no or over-the-counter medications; moderate (1.3%), requiring steroids; and severe (0.3%), if the reaction lasted over 30 days or required explantation. The authors suggest avoidance of CA in patients with known allergy to CA (such as used in application of prosthetic eyelashes and fingernails), in patients with multiple contact allergies, and in patients with skin conditions like psoriasis or atopic dermatitis. Interestingly, previous treatment with CA was not predictive of development of hypersensitivity. Careful removal of the delivery catheter to avoid leaving adhesive in the subcutaneous tissue may be protective against hypersensitivity and can be achieved simply by withdrawing the delivery catheter into the access sheath prior to removal of the entire apparatus as suggested by Sermsathanasawadi and colleagues.45 It should be remembered that CA is a permanent implant and will produce a foreign body reaction, albeit usually mild and localized.46 Clinically relevant granulomas are uncommon and generally related to extravasated adhesive on withdrawal of the delivery catheter.47
Conclusion
CA adhesive is overall a safe and effective method of achieving improvement in signs and symptoms of venous disorders with robust long-term occlusion rates. Adherence to instructions for use and avoiding use in patients with known allergic reactions, hypersensitivity, or immune compromise is important.

REFERENCES
1. Robertson L, Evans C, Boghossian S, Allan P, Ruckley V, Fowkes FGR. The incidence of chronic venous disease in the Edinburgh Vein Study. J Vasc Surg Venous Lymphat Dis. 2013;1:59-67.
2. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5):2S-48S.
3. Carradice D, Mazari FA, Samuel N, Allgar V, Hatfield J, Chetter IC. Modelling the effect of venous disease on quality of life. Br J Surg. 2011;98(8):1089-1098.
4. Biemans AA, Van Den Bos RR, Nijsten T. Endovenous therapies of varicose veins: indications, procedures, efficacy and safety. G Ital Dermatol Venereol. 2010;145(2):161-173.
5. Samuel N, Wallace T, Carradice D, Mazari FAK, Chetter IC. Comparison of 12-w versus 14-w endovenous laser ablation in the treatment of great saphenous varicose veins: 5-year outcomes from a randomized controlled trial. Vasc Endovascular Surg. 2013;47(5):346-352.
6. Gauw SA, Lawson JA, van Vlijmen-van Keulen CJ, Pronk P, Gaastra MTW, Mooij MC. Five-year follow-up of a randomized, controlled trial comparing saphenofemoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anesthesia. J Vasc Surg. 2016;63(2):420-428.
7. Rasmussen L, Lawaetz M, Bjoern L, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg. 2013;58(2):421-426.
8. Lawaetz M, Serup J, Lawaetz B, et al. Comparison of endovenous ablation techniques, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Extended 5-year followup of a RCT. Int Angiol. 2017;36(3):281- 288.
9. Proebstle TM, Alm J, Gockeritz O, et al. Five-year results from the prospective European multicentre cohort study of radiofrequency segmental thermal ablaton for incompetent great saephnous veins. Br J Surg. 2015;102:212-218.
10. Morrison N, Gibson K, Vasquez M, et al. VeClose trial 12-month outcomes of cyanoacrylate closure versus radiofrequency ablation for incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2017;5(3):321- 330.
11. Wittens C, Davies AH, Bækgaard N, et al. Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49(6):678-737.
12. Forlee MV, Grouden M, Moore D, Shanik G. Stroke after varicose vein foam injection sclerotherapy. J Vasc Surg. 2006;43:162-164.
13. Morrison N, Gibson K, McEnroe S, et al. Randomized trial comparing cyanoacrylate embolization and radiofrequency ablation for incompetent great saphenous veins (VeClose). J Vasc Surg. 2015;61(4):985-994.
14. Proebstle TM, Alm J, Dimitri S, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc surgery Venous Lymphat Disord. 2015;3(1):2-7.
15. Joh JH, Lee T, Byun SJ, et al. A multicenter randomized controlled trial of cyanoacrylate closure and surgical stripping for incompetent great saphenous veins. J Vasc Surg Venous and Lym Dis. 2022;10(2):353-359.
16. Almeida JI, Javier JJ, Mackay E, Bautista C, Proebstle TM. First human use of cyanoacrylate adhesive for treatment of saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1(2):174-180.
17. Proebstle TM, Alm J, Dimitri S, et al. The European multicenter cohort study on cyanoacrylate embolization of refluxing great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2015;3(1):2-7.
18. Kolluri R, Gibson K, Cher D, Madsen M, Weiss R, Morrison N. Roll-in phase analysis of clinical study of cyanoacrylate closure for incompetent GSVs. J Vasc Surg Venous Lymphat Dis. 2016;4:407- 415.
19. Gibson K, Morrison N, Kolluri R, et al. Twenty-four month results from a randomized trial of cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. 2018;6(5):606-613.
20. Morrison N, Kolluri R, Vasquez M, Madsen M, Jones A, Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36-Month outcomes of the VeClose randomized controlled trial. Phlebology. 2019;34(6):380-390.
21. Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized controlled trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Dis. 2020;8(6):978-989.
22. Chan YC, Law Y, Cheung GC, Ting AC, Chen SW. Cyanoacrylate glue used to treat great saphenous reflux: Measures of outcome. Phlebology. 2017;32(2):99- 106.
23. Hwang JH, Park S, Kim K, et al. Regression of varicose veins after cyanoacrylate of incompetent great saphenous veins without a localized concomitant procedure. J Vasc Surg Venous Lymphat Dis. 2019;7(3):375-381.
24. Gibson K, Minjarez R, Gunderson K, Ferris B. Need for adjunctive procedures following cyanoacrylate closure of incompetent great, small and accessory saphenous veins without the use of postprocedure compression: Three-month data from postmarket avaluation of the VenaSeal System (the WAVES Study). Phlebology. 2019;34(4):231-237.
25. Lane T, Kelleher D, Moore H, Franklin I, Davies A. Cyanoacrylate glue for the treatment of GSV incompetence in the anticoagulated patient. J Vasc Surg Venous Lymphat Dis. 2013;1:298-300.
26. Yang G, Parapini M, Gagnon J, Chen J. Comparison of cyanoacrylate embolization and radiofrequency ablation for the treatment of varicose veins. Phlebology. 2019;34(4):278-283.
27. Chan YC, Cheung GC, Ting AC, Cheng SW. Modification of protocol with one extra drop of endovascular cyanoacrylate improved closure rates in incompetent GSV. Phlebology. 2022 26 Mar. Epub ahead of print. doi:10.1177/02683555221082358.
28. Tang TY, Yap CJ, Soon SX, Chan SL, Choke ET, Chong TT. One-year outcome using cyanoacrylate glue to ablate truncal vein incompetence: a Singapore VenaSeal real-world post-market evaluation study (ASVS). Phlebology. 2021;36(8):609-619.
29. Park I. Successful use of VenaSeal system for the treatment of large GSV of 2.84cm diameter. Ann Surg Treat Res. 2018;94(4):219-221.
30. Vicente-Jimenez S, Lopez-Fernandez E, Carrasco P, et al. Clinical results and cost effectiveness of radiofrequency and cyanoacrylate compared with traditional stripping for treating varicose veins. J Vasc Surg Venous Lymphat Disord. 2021 Nov 12;S2213-333X(21)00523-0. doi:10.1016/j.jvsv.2021.10.015.
31. Toonder IM, Lam Y, Lawson J, Wittens C. Cyanoacrylate adhesive perforator embolization (CAPE) of incompetent perforating veins of the leg. A feasibility study. Phlebology. 2014;29:49-54.
32. Gibson K. Cyanoacrylate closure of perforator veins: initial technical feasibility and safety and presentation of a case of its use in a patient with Ehler’s Danlos syndrome and a nonhealing ulcer. Paper presented at American College of Phlebology Annual Congress; Nov 10 2018; Nashville, TN.
33. Mordhorst A, Yang G, Chen J, Lee S, Gagnon J. Ultrasound-guided cyanoacrylate injection for the treatment of incompetent perforator veins. Phlebology. 2021;36(9):752-760.
34. Kiguchi M, Reynolds K, Cutler B, et al. The need for perforator treatment after VenaSeal and ClosureFast endovenos saphenous vein closure in CEAP 6 patients. J Vasc Surg Venous Lymphat Dis. 2021;9:1510-1516.
35. Eroglu E, Yasim A. A randomized clinical trial comparing N-Butyl cyanoacrylate, radiofrequency ablation, and endovenous laser ablation for treatment of superficial venous incompetence. Two year follow up results. Eur J Vasc Endovasc Surg. 2018;56(4):553-560.
36. Calik E, Arslan U, Erkut B. Ablation therapy with cyanoacrylate glue and laser for refluxing great saphenous veins – a prospective randomized study. Vasa. 2019.48:405-412.
37. Daylan A, Islamoglu F. Comparative analysis of the results of cyanoacrylate ablation and radiofrequency ablation in the treatment of venous insufficiency. J Vasc Surg Venous Lymphat Dis. 2022;10(3):661-668.e2. doi:10.1016/j. jvsv.2021.09.001.
38. Premnath K, Joy B, Raghavendra V, Toms A, Sleeba T. Cyanoacrylate adhesive embolization and sclerotherapy for primary varicose veins. Phlebology. 2018;33(8):547-557.
39. Cho S, Gibson K, Lee SH, Kim S-Y, Joh JH. EGIT Incidence, classification, and risk factors of endovenous glue-induced thrombosis after cyanoacrylate closure of the incompetent saphenous vein. J Vasc Surg Venous Lymphat Disord. 2020;8(6):991-998.
40. Dimech A, Cassar K. Efficacy of cyanoacrylate glue ablation of primary truncal varicose veins compared to existing endovenous techniques: a systematic review of the literature. Surg J (N Y). 2020;6:e77-e86.
41. Chan S, Chan Y, Walsh S, et al. Endovenous cyanoacrylate ablation for chronic venous insufficiency and varicose veins among Asians. Ann Acad Med Singap. 2021;50:241-249.
42. Park I, Jeong M, Park C, Park W, Park E, Joh J. Clinical features and management of “phlebitis-like abnormal reaction” after cyanoacrylate closure for the treatment of incompetent saphenous veins. Ann Vasc Surg. 2019;55:239-245.
43. Fiengo L, Gwozdz A, Tincknell L, Harvey V, Watts T, Black S. VenaSeal closure despite allergic reaction to n-butyl cyanoacrylate. J Vasc Surg Cases Innov Tech. 2020;6(2):269-271.
44. Gibson K, Minjarez R, Rinehardt E, Ferris B. Frequency and severity of hypersensitivity reactions in patients after VenaSeal treatment of superficial insufficiency. Phlebology. 2020;35(5):337-344.
45. Sermsathanasawadi N, Pruekprasert K, Chinsakchai K, Wongwanit C, Ruangsetakit C. Cyanoacrylate granuloma after cyanoacrylate closure of incompetent saphenous veins. Dermatol Surg. 2021;47(10):1372-1375.
46. Almeida JI, Murray SP, Romero ME. Saphenous vein histopathology 5.5 years after cyanoacrylate closure. J Vasc Surg Venous Lymphat Disord. 2020;8(2): 280-284.
47. Parsi K, Kang M, Yang A, et al. Granuloma formation following cyanoacrylate glue injection in peripheral veins and AVM. Phlebology. 2020;35(2):115-123.
