Current status of thrombolysis for acute deep venous thrombosis
Anthony J. COMEROTA,
Marilyn H. GRAVETT
Jobst Vascular Center
University of Michigan
The Toledo Hospital, OH, USA
ABSTRACT
An important question involving the management of patients with acute deep venous thrombosis (DVT) is whether thrombus removal improves outcome, either by reducing postthrombotic morbidity or reducing recurrence rates. Eliminating thrombus is intuitively appealing since persistent thrombus causes venous obstruction and destroys valve function, leading to ambulatory venous hypertension and, ultimately, symptoms of the postthrombotic syndrome. Natural history studies of acute DVT have demonstrated that when spontaneous lysis of thrombus occurs, valve function is preserved, especially when lysis occurs within 1-2 months of diagnosis. Early in the course of the development of thrombolytic agents, investigators evaluated the use of systemic thrombolysis for management of patients with acute DVT. While observations were made that postthrombotic morbidity was reduced, failure rates remained high and bleeding complications increased. Intrathrombus delivery of plasminogen activators facilitated thrombus resolution and reduced bleeding complications. This manuscript reviews the evolution of thrombolytic therapy for patients with acute DVT and addresses the integration of pharmacomechanical techniques and the development of new pharmacologic agents.
The standard recommendation for treatment of patients with acute DVT is antithrombotic therapy, beginning with heparin (intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin), followed by oral anticoagulation with warfarin.1 Therapeutic anticoagulation prevents thrombus extension, pulmonary embolism (PE), and death from PE, and reduces the risk of recurrent venous thrombosis. Elastic compression stockings are recommended to reduce the risk of postthrombotic syndrome. The majority of patients with symptomatic DVT have involvement of the popliteal vein, the femoral vein, or more proximal veins.2 Approximately 50% of patients with proximal DVT develop symptoms of the postthrombotic syndrome, and in 25% of patients with proximal DVT, the chronic postthrombotic complications are severe.3 Two well-designed randomized trials have demonstrated that wearing 30- 40 mm Hg compression stockings reduces the risk of postthrombotic morbidity by approximately 50%.3,4
Postthrombotic complications of acute DVT are the result of persistent venous obstruction and destruction of vein valve function.5,6 Natural history studies of acute DVT have demonstrated that when spontaneous lysis of thrombus occurs, valve function is preserved, especially when lysis occurs within 1 to 2 months of diagnosis.7 It seems intuitive that adopting a strategy that eliminates acute thrombus in patients presenting with acute DVT would significantly reduce postthrombotic morbidity.
Early in the course of the development of thrombolytic agents, investigators evaluated the use of systemic thrombolysis for management of patients with acute DVT. While observations were made that postthrombotic morbidity was reduced, failure rates remained high and patients paid the price of significantly increased bleeding.8 Iliofemoral DVT is associated with the most severe postthrombotic morbidity. The percentage of iliofemoral DVT patients suffering postthrombotic complications and the severity of those complications substantially exceed the postthrombotic complications occurring in patients with infrainguinal DVT. These individuals represent a valid subset of patients with acute DVT who can justifiably be approached with a strategy of thrombus removal. The evolution of management of these patients has demonstrated the value of intrathrombus delivery of plasminogen activators to speed thrombus resolution and reduce bleeding complications.
This manuscript will review the development of thrombolytic therapy for patients with acute DVT and follow its evolution to catheter-directed thrombolysis and the integration of pharmacomechanical techniques. In addition, we will briefly address the development of new pharmacologic agents.
MECHANISM OF THROMBOLYSIS
Clot dissolution occurs as a result of plasminogen being activated by one of a number of plasminogen activators to form plasmin, which is the active enzyme. Plasminogen circulates as an inactive zymogen. In the systemic circulation, free plasminogen circulates as GLUplasminogen. When thrombosis occurs, plasminogen binds to fibrin and is altered, forming LYS-plasminogen, which is more susceptible to activation by a plasminogen activator. Alkjaersig and colleagues9 demonstrated that the basic mechanism of clot lysis by plasminogen activators is the activation of fibrin-bound plasminogen within thrombus to form plasmin, which then actively dissolves the clot. When plasmin escapes into the systemic circulation, it is instantaneously neutralized by _2-antiplasmin. Low doses of plasminogen activators are neutralized in the systemic circulation by plasminogen activator inhibitors. Pharmacologic doses of plasminogen activators persist in the circulation since they supersaturate the plasminogen activator inhibitors.
Since a relatively small percentage of systemicallyinfused plasminogen activators will come in contact with thrombus to activate fibrin-bound plasminogen, it is understandable that systemic therapy will be less effective than techniques that directly deliver plasminogen activator into the thrombus, thereby maximally activating fibrin-bound plasminogen.
Systemic thrombolytic therapy for acute DVT
The initial thrombolytic treatment of acute DVT was performed systemically, with plasminogen activators infused intravenously. Between 1968 and 1990, 13 studies of systemic thrombolysis versus anticoagulation for acute DVT were reported. These studies used ascending phlebography for initial diagnosis and to evaluate lytic success following lysis (Table I).10-25
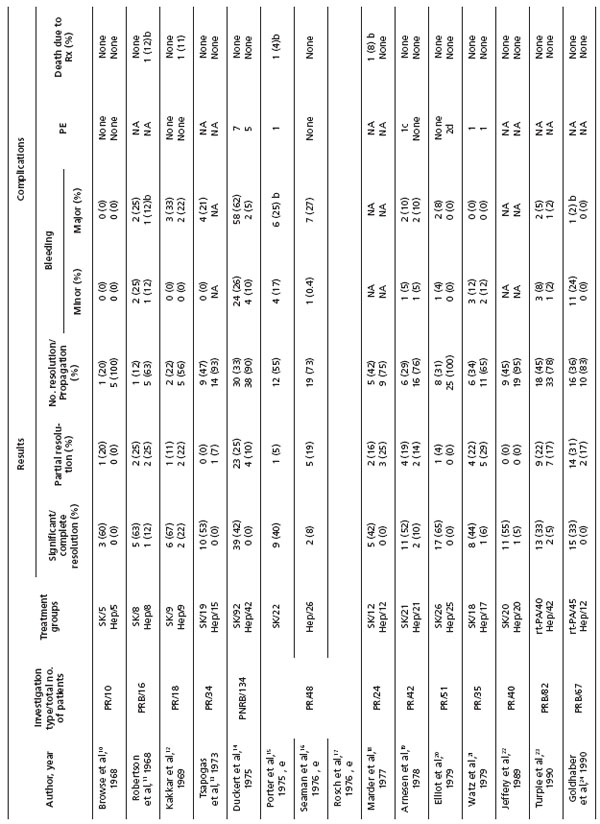
Table I. Review of anticoagulation versus systemic lytic therapy for deep vein thrombosis (Reprinted with permission from Elsevier)25
An overall analysis of these studies demonstrates that 45% of thrombolysis patients had significant or complete clearing of their thrombus compared with 4% of those treated with anticoagulation alone.8 Furthermore, the majority (82%) of anticoagulated patients had no clearing or actually worsened.
Elliot et al20 and Arnesen et al26 conducted trials of systemic thrombolysis in 78 patients, using the lytic agent streptokinase in similar protocols, and followed them up long term (1.6 and 6.5 years, respectively). Analysis of their outcomes demonstrates that postthrombotic symptoms occurred most frequently and with the greatest severity in patients treated with anticoagulation alone.
A meta-analysis of systemic thrombolytic therapy for acute DVT reported improved clot dissolution at an increased risk for bleeding.8 Subsequently, a Cochrane analysis of randomized trials of thrombolysis for acute DVT vs anticoagulation reported that complete clot lysis occurred significantly more often in patients treated with thrombolysis when examined at both early and late follow-up.27 Significantly less postthrombotic morbidity was reported in lytic patients. Although venous ulcers were reduced and valve function improved, the numbers of patients evaluated for these end points were small, and so differences did not reach significance. Bleeding complications occurred more frequently in the thrombolytic group.
Although systemic thrombolysis is associated with better outcomes than anticoagulation alone, failures rates are still high. A likely cause of poor outcome in these studies is the failure of contact of the plasminogen activator with fibrin-bound plasminogen within the thrombus. A technique designed to ensure maximal exposure of fibrin-bound plasminogen to the plasminogen activator will be associated with improved outcomes.
Catheter-directed thrombolysis
The rationale for catheter-directed thrombolysis (CDT), which was initially used in patients with acute arterial occlusion,28 is that rapid lysis is achieved with lower doses of thrombolytic therapy, resulting in fewer serious bleeding complications. Furthermore, positioning the catheter directly into the thrombus (intrathrombus delivery) takes advantage of the basic mechanism of thrombolysis, which is activation of fibrin-bound plasminogen.9
Numerous studies have reported good outcomes from catheter-directed thrombolysis for acute DVT (Table II).29-47 Their outcomes have been summarized and published previously.25 Three larger reports in particular showed a success rate of approximately 80%.32,33,48
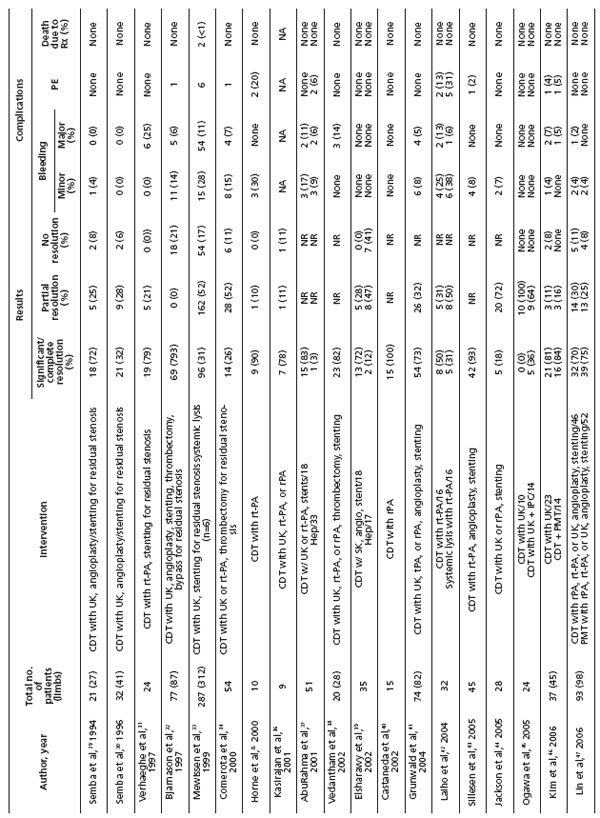
Table II. Review of catheter-directed thrombolysis for acute deep vein thrombosis (Reprinted with permission from Elsevier)
Quality of life (QOL) is improved in patients with iliofemoral DVT treated with CDT. This was shown in a study evaluating iliofemoral DVT patients treated with CDT and compared with a contemporaneous group of iliofemoral DVT patients treated with anticoagulation alone.49 The improved QOL directly correlated with successful lysis. Patients failing lytic therapy had the same QOL as patients treated with heparin.
Correction of underlying venous lesions or residual stenoses following successful thrombolysis was shown to contribute to long-term success. In the National Venous Registry, 74% of limbs treated with stent placement following lysis remained patent at 1 year, compared with 53% of limbs without stent placement (P<0.001). This observation most likely underscores the importance of correcting an underlying iliac vein stenosis (May- Thurner). Significant bleeding complications were reported in 9.9% of patients, with the majority being related to the catheter puncture site. Intracranial hemorrhage was reported in 2 patients (0.25%). Reports published during the past five years have shown a bleeding complication rate less than half (4.1%) that in earlier reports. This is likely due to more appropriate patient selection and experience with the technique. The reporting of pulmonary embolism (PE) was limited to symptomatic PE, which occurred in 4 patients (0.5%) and was fatal in one.
Unfortunately, there are few data from randomized trials of catheter-directed thrombolysis versus standard anticoagulation alone. Elshawary et al39 performed a small study randomizing 35 patients with iliofemoral DVT to a pulse-spray catheter-directed thrombolysis with streptokinase versus anticoagulation alone. A six-month follow-up demonstrated significantly better patency and significantly less valvular reflux in patients receiving catheter-directed thrombolysis (P<0.001, P=0.04, respectively). Enden et al50 reported an ongoing trial of 200 patients being randomized to CDT with tissue plasminogen activator (tPA) versus routine anticoagulation with low-molecular-weight heparin and coumadin in Norway. Approximately half of the 200 patients have been randomized to date. The primary outcome is patency of the iliofemoral venous segment at 6 months and the incidence of postthrombotic syndrome at 2 years. Other objectives will evaluate bleeding, health-related QOL, cost-effectiveness of therapy, procedural success, patency at 2 years, and postthrombotic syndrome at 6 months and 5 years.
A much larger 1000-patient trial is currently being evaluated for funding by the United States National Institutes of Health. This study will incorporate patients with proximal infrainguinal DVT as well as iliofemoral DVT randomized to CDT incorporating pharmacomechanical techniques plus anticoagulation versus anticoagulation alone. The results of these randomized trials are anxiously awaited and will most certainly guide future care of these patients with extensive venous thrombosis.
When successful, catheter-directed thrombolysis can relieve obstruction, maintain valve function, and preserve QOL.42,43,49 However, the technique has been associated with prolonged infusion times, large doses of plasminogen activators, and the potential for bleeding complications. In the National Venous Registry, average urokinase dose was 7.8 million units with a mean infusion time of 53.4 hours. To address these concerns, adjunctive techniques designed to facilitate thrombolysis have been developed in recent years. These include percutaneous mechanical and pharmacomechanical devices that macerate and/or aspirate thrombus particles from the venous system.
Percutaneous mechanical thrombectomy and pharmacomechanical thrombolysis
Percutaneous mechanical venous thrombectomy was put into proper perspective by Vedantham et al38 in their analysis of patients with acute DVT managed with mechanical thrombectomy in addition to other techniques, including CDT and balloon dilation and stenting. Percutaneous thrombectomy is unlikely to be effective as the only management option for patients with acute lower extremity DVT. Only 26% of the thrombus calculated by quantitative venography was removed, as analyzed in a retrospective study. The addition of catheter-infused plasminogen activator significantly improved results.38 Overall success was that which could have been anticipated with CDT alone. The investigators thought the overall treatment time with the pharmacomechanical technique was shortened, although both on-table procedure time and the cost of the procedure increased. Others have noted similar observations that mechanical thrombectomy alone has unsatisfactory results that can be substantially improved with the addition of a plasminogen activator.36 Pharmacomechanical thrombolysis combined with catheter-directed thrombolysis was the technique used with good results and no major complications in the randomized trial comparing CDT with anticoagulation.39
Pulmonary emboli have been observed with percutaneous mechanical thrombectomy. In a small series of 18 patients treated with a rotational thrombectomy device for iliocaval DVT following placement of a vena caval filter, all patients experienced oxygen desaturation and hypoxemia during the procedure, and 5 were found to have thrombus trapped within the caval filter. There was one procedure-related death (6%)51
Pulse-spray pharmacomechanical thrombolysis of clotted hemodialysis grafts demonstrated an 18% incidence of PE in patients treated with a plasminogen activator pulse-spray solution compared with a 64% incidence of PE in patients treated with a heparinized saline pulsespray solution (P<0.04).52 Because hemodialysis grafts are in direct communication with the venous circulation, embolic complications in these patients should be similar to those with proximal acute DVT. However, embolic complications with mechanical devices alone might be magnified when treating thrombus in much larger veins such as the iliofemoral venous system.
Segmental pharmacomechanical thrombolysis is an increasingly popular method in patients with acute DVT. One of the more promising pharmacomechanical catheter systems is the Trellis® catheter (Bacchus Vascular, Santa Clara, CA). This double-balloon catheter allows segmental infusion of a relatively high concentration of plasminogen activator per unit time and volume of thrombus treated. Thrombus is segmentally treated between two balloons. The proximal and distal balloons restrict the plasminogen activator to the segment of thrombus being treated. The intervening catheter assumes a spiral configuration and, when activated, spins at 1500 rpm. After a 15-20 minute treatment cycle, the liquefied and particulate debris is aspirated. Repeat phlebograms evaluate lytic success and whether treatment should be repeated or whether additional vein segments should be treated (Figure 1). Preliminary observations demonstrate rapid resolution of the thrombus treated with minimal exposure of the systemic circulation to the thrombolytic agent.
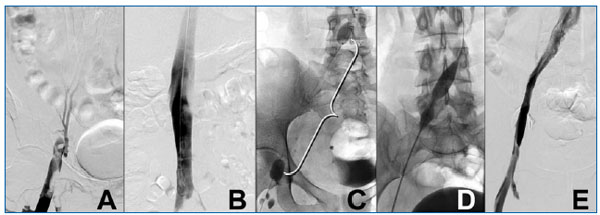
Figure 1. A 26-year-old woman presents with a two-day history of severe left lower extremity pain and edema extending from the toes to the upper thigh. A venous duplex exam suggested iliac vein thrombosis. (A) The ascending phlebogram (prone position) demonstrates left common femoral, external iliac, and common iliac vein thrombosis. (B) The vena cava was patent. Isolated segmental pharmacomechanical thrombolysis with a double-balloon catheter (Trellis®) was used. (C) The proximal and distal balloons are visualized. Three mg of tissue plasminogen activator was injected in a volume of 6 cc of fluid. A single 20-minute run of the intervening catheter rotating at 1500 rpm resolved the thrombus and revealed a residual common iliac vein stenosis. (D) A balloon angioplasty was performed and the stenotic “waist” is visualized (arrow). A stent resolved the stenosis. (E) A completion phlebogram demonstrates complete thrombus resolution and unobstructed venous drainage into the vena cava. The patient was discharged and has been symptom-free.
Ultrasound-accelerated thrombolysis is another interesting adjunctive technique for catheter-directed thrombolysis. The LYSUS catheter system (EKOS Corp, Bothell, WA) integrates a lytic infusion catheter with multiple ultrasound-emitting foci along the length of the catheter. The ultrasound waves distort and fragment fibrin, increasing its surface area and improving the rapidity of activation of fibrin-bound plasminogen, which increases the speed of lysis. Preliminary observations on both the arterial and venous sides of the circulation suggest that this unique method of pharmacomechanical thrombolysis reduces treatment times, thereby reducing potential complications.
New pharmacologic agents
In addition to new developments in the mechanical delivery of plasminogen activators to thrombus and the mechanical management of thrombus itself, a new class of pharmacologic agents that directly lyse clots without requiring a plasminogen activator is being developed. These agents appear to act more rapidly than traditional plasminogen activators and are immediately neutralized upon entering the systemic circulation.
Alfimeprase and plasmin are two such agents. They are direct fibrinolytic agents that are not plasminogendependent and are not inactivated by plasminogen activator inhibitor-1.
Alfimeprase is a direct-acting lytic agent which is a fibrinolytic zinc metalloproteinase, which is a truncated form of fibrolase. It is derived from southern copperhead snake venom and is produced by recombinant techniques in Pichia pastoris. Alfimeprase is instantly neutralized in the systemic circulation by _2- macroglobulin. Although no studies have been performed in patients with acute DVT, intrathrombus infusion has been studied in patients with acute arterial occlusion. Phase 1 and phase 2 studies demonstrated safety, but phase 3 trials demonstrated no benefit of catheter-directed alfimeprase versus placebo in patients with acute arterial and graft occlusion.
Plasmin is also a direct-acting fibrinolytic agent. It is produced from pooled plasma. It is instantly neutralized by _2-antiplasmin when it escapes into the systemic circulation. Animal studies have demonstrated improved efficacy compared with recombinant tPA (rtPA) in models of acute aortic occlusion.53-55 Reduced distant bleeding compared with rtPA has also been observed. The explanation for improved safety of plasmin is that antiplasmins rapidly neutralize plasmin that escapes into the circulation, whereas rtPA circulates, binds to hemostatic plugs at sites of vascular injury, degrades fibrin, and thereby induces new hemorrhage. Plasmin is currently in phase 1/2 trials for acute arterial and graft occlusion, with plans for study in patients with acute DVT.
CONCLUSION
Available evidence supports the use of CDT in patients with extensive, symptomatic acute DVT. Bleeding complications have been reduced, especially during the past five years. The improvement in technology allowing the incorporation of mechanical and pharmacologic techniques has improved the speed of lysis, reduced treatment times, and reduced doses of plasminogen activators.
The development of a new class of thrombolytic agents that act directly on thrombus without the need for a plasminogen activator and are rapidly neutralized upon entry into the systemic circulation holds promise of further improving outcomes and reducing risks of bleeding complications.

