Critical Role of the Vasa Venarum in the Pathogenesis of Chronic Venous Disease.Part II: Therapeutic Implications
Dominik R. WEISS2,
Gerd JUCHEM3,
Hugo PARTSCH4
2 Department of Transfusion Medicine and Hemostaseology, University of Erlangen-Nuremberg (FAU), 91054 Erlangen, Germany
3 Department of Cardiac Surgery, University of Munich (LMU), 81377 Munich, Germany
4 Emeritus Professor of Dermatology, Medical University of Vienna, Austria
ABSTRACT
Standing or sitting for a long duration is accompanied by venous distension and pooling of blood in the larger leg veins and the lower vena cava. Under these conditions, reflux from the vein lumen into the nutritive microvascular system of the vein wall can occur. Resulting acute inflammatory processes lead to a breakdown of the endothelial barrier in the corresponding venules, edema formation, and venular thrombosis, events that have the potential to spread into the lumen of the respective parent vein. To forestall the development of chronic venous insufficiency and obviate the dangers of venous thrombosis and post-thrombotic syndrome, all measures aimed at improving venous return as well as the perfusion and tightness of the nutritive microcirculatory system in the vein wall are welcome. The most important treatment modalities to achieve this goal are compression, surgical intervention, and certain pharmacological measures that will be addressed and discussed against the background of their effectiveness and the current literature.
INTRODUCTION
As described in Part I, standing or sitting for a long duration is accompanied by venous distension and pooling of blood in the larger veins of the lower extremity, the pelvis, and the lower vena cava. Blood emerging from the microcirculatory beds of the leg organs must now mix with the pooled blood in the draining veins. This decreases the concentrations of unstable antithrombogenic mediators added to the blood from the microcirculatory endothelium (as is the case while lying or running). In addition, the high hydrostatic pressures in the capillaries and venules during standing increase the filtration of fluid, resulting in “hydrostatic edema,” an increase of the local hematocrit and a compensatory increase of lymph drainage. Should such a situation become chronic, the lymph drainage will decompensate and inflammatory processes can superimpose themselves on this hydrostatic background. Inflammatory mediators, synthesized and released locally, can elicit active contraction of the venular endothelial cells, thus strongly enhancing the exit of plasma into the interstitium. During this “inflammatory edema” formation, the vessel walls will be substantially remodeled and a final thrombotic occlusion of the lumina may occur.
Stasis in the microcirculation correlates with elevated pressure in the veins. As a consequence of gravity, this may happen in immobile patients who spend their lives in wheelchairs, in extremely obese patients with restricted mobility, or in patients with massive venous refluxes leading to ambulatory venous and capillary hypertension while walking. Due to steadily increasing pressure in the venules, there is an increase of the transcapillary pressure gradient and a decrease in the shear stress on the venular and capillary endothelium. Low shear stress promotes leukocyte adhesion and penetration into the endothelium, which triggers an inflammatory reaction,1 as described in Part I.
In this situation and in the context of the novel pathophysiological insights described above (particularly in Part I), all measures aimed at improving venous return are welcome. The most important treatment modalities to achieve this goal are compression, surgical interventions, and pharmacological measures that will be addressed briefly in the following article.
COMPRESSION THERAPY
The most important mechanism of compression treatment of the lower extremities is to counteract gravity as the major hindrance affecting venous return while in the upright position.
In the lower extremities, sitting or standing for long periods of time leads to an increased shift of fluid from the microcirculation into the tissue causing the so called physiological “evening edema” or “occupational edema.” After a long day, shoes may no longer fit and an unpleasant feeling of tension in the legs may occur. Compression is very effective at preventing this hydrostatic edema and reducing subjective complaints. It could be demonstrated that compression stockings in a pressure range of around 20 mm Hg are able to prevent occupational edema and alleviate symptoms.2 By measuring the capillary filtration rate, compression stockings with higher stiffness are superior to highly elastic products with low stiffness.3 The prevention of leg edema after long flights, by using stockings, may also have a thromboprophylactic effect by preventing the increase of local hematocrit in leg veins occurring without compression after sitting for long periods due to increased filtration of fluid into the tissue.4 Compression is also able to reduce manifest edema, which can be achieved with relatively low pressures in patients with pitting edema before it becomes indurated.5 Reduction in skin edema is associated with an increase in capillary density.6
Chronic venous insuffiency (CVI) is characterized by a failure of the venous pump mechanism (see Part I , Figure 2), in association with venous reflux and/or venous obstruction. As demonstrated in numerous studies, compression reduces venous reflux and improves the venous pump. A prerequisite for this mechanism is a venous caliber reduction, which can be demonstrated by using magnetic resonance imaging, not only in the supine, but also in the standing position7 (Figure 1). Together with Duplex-investigations, such studies were useful to find the minimal compression pressure required to narrow the leg veins in the upright position.8 In patients with CVI, inelastic compression applied with a pressure >50 mm Hg reduces pathological reflux in both superficial and deep veins,9 increases the ejection fraction of the venous pump,10 and reduces ambulatory venous and capillary hypertension.11,12 Pressure measurements in dorsal foot veins, performed in patients with ulcers due to a congenital absence of venous valves, showed an improvement in ambulatory venous hypertension by compression, disproving the popular concept that compression works by making distended valves competent again.13
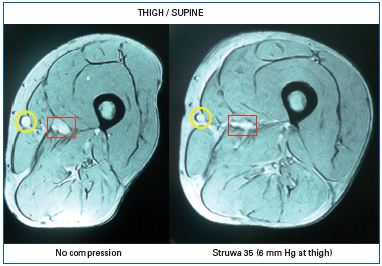
Figure 1: Cross section at mid-thigh level in a supine
patient with a large great saphenous vein (yellow circle)
obtained by magnetic resonance imaging.
Left without compression, right with a compression
stocking exerting a pressure of 6 mm Hg at thigh level.
Compression reduces the calibre of the superficial (yellow
circle) and deep vein (red square) and leads to a more
circular configuration of the thigh.7
The most severe form of chronic venous hypertension, which leads to a massive inflammatory reaction in the context of CVI, is the venous leg ulcer, for which compression is the cornerstone of management. Biopsies taken from the periphery of the ulcer and from healthy tissue have shown that compression treatment can reduce the raised levels of diverse inflammatory cytokines in the ulcer region, while TGF is upregulated.14 Additional studies, employing noninvasive confocal laser scanning microscopy in ulcer patients, have shown an increased cell flow rate in dermal capillaries and a reduction in the number of inflammatory cells after treatment with inelastic Putter bandages when compared with local therapy without compression.15 In addition, 4 weeks of compression therapy have been shown to enhance the expression of tight junction molecules in the vascular endothelium, which may play a role in preventing extracellular edema.16
In patients with mixed arterial-venous ulcers and concomitant peripheral arterial occlusive disease, inelastic compression bandages applied with a pressure of 17 Previous experimental studies, in healthy persons, have also reported an increase in arterial blood flow beneath a compression bandage at pressures up to 40 mm Hg.18 Myogenic relaxation of the arterial wall, release of vasodilator mediators, and a reduction in the arteriovenous pressure gradient following improvement in the venous pump function are discussed as possible explanations. Nutritive microvessels in large artery and vein walls may play an important role in both myogenic relaxation of the arterial wall and release of vasoactive substances.
In the microcirculation, intermittent compression produced by walking with inelastic compression or with pumps will increase shear forces at the vessel walls and reduce leukocyte adhesion to the endothelium and increase release of diverse antithrombotic, antiinflammatory, and vasodilatory mediators from the endothelium (eg, various platelet inhibitors, anticoagulants such as tissue factor pathway inhibitor [TPFI], and profibrinolytic tissue plasminogen activator [tpA], (see Part I, Figure 3). This latter effect has been demonstrated during intermittent compression using appropriate pumping systems.19
An acceleration of venous blood flow velocity in the legs counteracting stasis is an important mechanism of action to prevent thrombosis. This can be achieved by either pneumatic pumps that squeeze the legs intermittently or by sustained compression20.
In the supine position, reduction in the cross-sectional area with unchanged volume inflow will raise the linear venous flow velocity in the compressed parts of the leg (Figure 1). This could be demonstrated by measuring venous transit times with and without thromboprophylactic stockings using intravenous injections of tiny amounts of radioactive tracers.21 Blood will arrive more rapidly in the lungs (see Part I, Figure 2), where the enormous increase in the endothelial surface and hence the pivotal “clearance function” of the pulmonary endothelium comes into play. The continuous removal of activated coagulation factors, vasoactive compounds, and inflammatory mediators that eventually appear in the mixed venous blood is a complex function of the lungs that is certainly as vital as gas exchange.
Inflammatory processes in the vein wall obviously play prominent roles in the pathogenesis of venous thrombosis22,23 (Part I). In patients with superficial thrombosis, used as a model for deep vein thrombosis (DVT), excisions of thrombosed vein segments have been performed after intravenous injection of radioactively labeled 131I-fibrinogen. The concentration of radioactivity was much higher in the adventitia and the rest of the vein wall, rather than in the clot itself.24 This means that the substrate for a “positive fibrinogen uptake test,” as used for the detection of DVT, is little to no accumulation of fibrin in the clot and far more accumulation in the vein wall (Figure 2). These findings support the concept that venous thrombosis is, in fact, more complex than the formation of an intraluminal thrombus, and highlights the currently proposed importance of the venular endothelial barrier and the pericytes within the vasa venarum network. The accumulation of fibrin in such experiments is unlikely to be attributable to simple diffusion of fibrinogen from the lumen into the wall periphery due to the large distance from the vein lumen, the tight internal elastic lamina, and the whole media of the adventitia. Rather, the observed deposition of a surprisingly large amount of fibrin, particularly in the layer of the vessel wall enclosing the thrombus, is most likely the substrate of a massive thrombotic reaction occurring in the vasa venarum network and in the interstitial space surrounding the corresponding microvessels, which drain into the lumen of the respective parent vein. Hemostatic reactions are always immediately followed by local activation of the acute immune defense. This inflammatory response in the vein wall, which is associated with deep vein thrombosis, can be visualized by gadolinium magnetic resonance venography. Gadolinium extravasates into tissues during inflammation, producing a perithrombus enhancement, which can be visualized by magnetic resonance imaging. The typical “bulls-eye sign” corresponding to gadolinium enhancement in the vein wall has been described as a helpful discriminator to differentiate between acute and chronic DVT.25
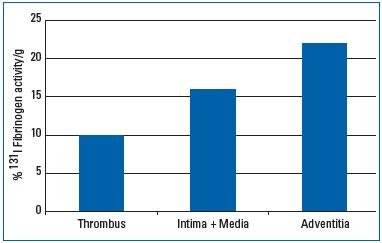
Figure 2: Radioactivity per 1 g of tissue measured in 3
patients with superficial leg vein thrombosis 24 hours after
intravenous injection of 131 I fibrinogen and excision of the
thrombosed vein segment. There is more fibrin in the vein
wall and especially in the perivenous tissue than in the
clot.24
Recent application of positron emission tomography after the uptake of radioactive fluorodeoxyglucose (18F-FDG) during acute DVT resulted in documentation of an increased metabolic activity in the wall of the respective thrombosed veins, which extended into the “adjacent soft tissues.”26 This result probably reflects the high inflammatory activity in and around the vena venarum networks, which are the densest in the adventitia and its surrounding connective tissue (Part I).
For such reasons, compression therapy considerations for future studies must address mechanical influences on the thrombus and preventive effects on coagulation and inflammatory reactions within the vein walls. Such mechanisms are endorsed by studies demonstrating that compression therapy reduces proinflammatory cytokine levels and increases the level of the antiinflammatory cytokine IL-1,14 and that increases in shear stress releases anti-inflammatory substances from the endothelial cells.19 Clinical research has demonstrated that during catheter directed thrombolysis, the use of intermittent pneumatic compression pumps in patients with proximal thrombosis achieves better early and late results compared with thrombolysis alone.27
In the past, patients with deep leg vein thrombosis were frequently immobilized due to fear of a pulmonary embolism. Controlled clinical studies have shown that immediate mobilization under adequate anticoagulation combined with compression, results in a more rapid reduction in swelling and pain than bed rest, without raising the risk of symptomatic pulmonary embolism.20 When deep vein thrombosis develops in a mobile patient, multiple and often clinically silent pulmonary emboli can be detected in a large proportion of patients, which justifies the term “venous thromboembolism.”28 In such cases, it could also be argued that it is better to shift small fresh parts of the thrombus under compression and walking into the pulmonary capillary system than to leave them in the leg veins to avoid the risk of causing irreversible damage to vein walls and valves, or of developing into large and possibly life-threatening emboli.29 The pulmonary microcirculatory system acts like a filter, and entrapped small thrombi are prone to prompt and complete lysis due to the extremely high ratio of endothelial surface area to blood volume and the profibrinolytic potency of the endothelial surface (see Part I, Figures 1 and 4). As a consequence, the most recent guidelines now recommend compression with both ambulatory exercise and optimal anticoagulation as the conservative basal therapy for deep leg vein thrombosis.20
Signs and symptoms that may occur as long-term complications of DVT are included under the term “Postthrombotic syndrome” (PTS), also called postphlebitic syndrome. Randomized controlled studies have documented a 50% reduction in the incidence of clinical signs and symptoms of a postthrombotic syndrome when compression stockings are worn for up to two years after an acute episode of deep vein thrombosis.30 PTS, which presents with clinical signs of chronic swelling, skin changes, and ulceration in the leg with a previous thrombosis, is a classic model for chronic inflammatory changes particularly in the vein wall, in which the pathological processes described above play a key role. The resulting pathology is determined not only by a variable proportion between persisting venous occlusion and recanalization of vein-segments with valve damage and reflux, but also by fibrotic changes in the vein walls, which undergo continuous remodeling.31 Calcification and even ossification of vein walls may be late signs of a previous, but sometimes asymptomatic thrombosis. A persistent inflammatory reaction initiated in the acute phase of DVT, may indeed be a more important risk factor for the development of PTS than hypercoagulability.32,33 Pathological consequences are initiated in the acute phase of DVT, which explains the importance of an exact anticoagulation treatment together with avoiding stasis by immediate compression to reduce the incidence of recurrent thrombotic events and to prevent PTS.Compression in the acute phase of DVT reduces pain and edema immediately34 and continuation of compression is able to maintain this situation and prevent PTS.35 If compression therapy is initiated too late, eg, several weeks after the acute phase of DVT, prevention of PTS may be less prominent. This is probably one explanation for the contradictory results of a recently published study that was unable to find differences between compression stockings and “placebo-stockings” regarding the development of signs and symptoms of PTS two years after proximal DVT.36 In particular, the very low compliance rate for wearing stockings and the restriction to weak, subjective outcome parameters make this study unreliable.
Radionuclide scintigraphy has shown that the deep, subfascial lymph transport is disturbed not only during acute thrombosis, but also over the period of a subsequent postthrombotic syndrome.37,38 It can be assumed that the mentioned fibrin deposition in the adventitia of occluded veins will also include the accompanying lymph collectors. In any case, 7 days of compression therapy with firm zinc paste bandages has been shown to consistently improve subfascial lymph transport.39 This result can be explained by stimulation of fibrinolysis during intermittent pneumatic compression due to the massaging effect achieved during walking exercises under stiff bandages40 and enhanced contractions of lymph collectors.
More precise concepts for the mechanism accounting for the clinically impressive effects of compression with respect to prophylaxis and treatment of PTS are still lacking. The effects of compression on the vessel walls and microcirculation have been widely neglected until now. The complex pathogenetic factors and selfpropelling pathomechanisms (Part I), open a wide scope for future histological, biochemical, and physiological studies.
ENDOVENOUS TECHNIQUES AND VASCULAR SURGERY
There is an ongoing dispute regarding the pathogenesis of varicose veins and their recurrence after treatment. In a simplified manner, this dispute involves two main concepts: (i) distal venous dilation (“descending, hemodynamic theory”) is mainly caused by reflux due to valvular incompetence; and (ii) the origin of the illness (“ascending theory”) is due to structural abnormalities in the vein wall. Based on the findings reported in Part I, both concepts involve cellular and molecular changes, which lead to histological alterations in the vein wall and the extracellular matrix. Actually, such changes in the vein wall and hemodynamic factors are linked together in a vicious circle.1,41
Regarding treatment options, the introduction of endovenous techniques has given new stimuli for the management of both venous reflux and proximal venous obstruction based on experience and a modern, anatomical/topological definition. Today, venous reflux can be followed downstream from the large venous trunks into the region of ulcers using Duplex ultrasound,42 and can be eliminated by endovenous procedures including foam sclerotherapy. Obstructions to upstream venous flow in the pelvic region can be recanalized using dilating catheter techniques and stents. In both cases, the treatment aims to eliminate the hemodynamic triggers for chronic venous hypertension. In practice, unfortunately, the decision for such intervention is frequently made too late, ie, after irreversible tissue damage has already occurred.
All ablative endovenous techniques aim to occlude refluxing veins. To reach this goal, thermal ablation of incompetent superficial veins by laser fibers, radiofrequency, or steam are applied. Another effective method is chemical ablation by injecting sclerosing agents into the respective veins. The result is always the destruction of the endothelial layer, which progresses to variable structural damage to the vessel wall and the vasa vasorum.43,44 Local thrombosis is initiated promptly after exposure to subendothelial pericytes (see Part I, Figure 3), which illustrates the newly recognized ubiquitous distribution of the prothrombotic pericytes in the different branches of the circulatory system. Ideally, the vein will finally be remodeled to a fibrotic cord. Ingrowing new vascular networks, a phenomenon which had been called “intraluminal neovascularization,”45 may originate from the nutritional vasculature in the outer layers of the treated vein. Due to this event, an unfavorable recanalization of the occluded vein may happen.
The critical involvement of pericytes in the development of venous disease described in part I of this publication has considerable impact on the outcome of classical varicose vein surgery. Pericytes nourish endothelial cells and are of central importance for angiogenic processes. In fact, after surgical ablation of saphenous veins, the pericyte-rich adventitial vasa venarum of the remaining stump and surrounding scar tissue are often involved in the development of progressive neovascularization, giving rise to the recurrence of varicosity.46
The influence of compression, specifically on the tissue changes initiated, by thermal or chemical vein ablation of refluxing veins, has been poorly investigated. Recent animal experiments were not successful in preventing reperfusion by compression (≤120 hours) after injection of sclerosing agents into rabbit ears.47
In the case of bypass operations or reconstruction of deep vein valves, measures that are sporadically performed in specialized centers only, care must be taken to ensure that the endothelium of the implanted vessel segments remain intact and undamaged.48,49 Basic research on the reendothelialization of different stent material concentrated on arterial circulation and animal experiments. Lots of research, especially with regards to venous stents, still needs to be conducted. Understanding the underlying pathophysiological mechanisms better, as described in Part I, will provide helpful stimuli for future research.
NEW PHARMACOLOGICAL ASPECTS IN THE THERAPY OF CVI
As described in Part I, CVI is a chronic disease, probably originating in the nutritive microvasculature in leg vein walls and/or leg organs, and in which local coagulation mechanisms and inflammatory reactions play a central, self-reinforcing role. It is clear that this is the site for intervention, both with effective and specific medications, and with the aim of inhibiting, or even permanently preventing, the chronic progression of the disease. The main benefit of physical or surgical therapies must be viewed in the context of prophylaxis or support: alone they are insufficient to prevent the spreading microcirculatory inflammatory processes. However, evaluation of venous drug therapy effectiveness is beset by diverse fundamental difficulties.50 For example, the objective assessment of certain symptoms, eg, pain and edema, is difficult. In addition, quantitative assessment of fluid accumulation in the legs, a distinction should be made between hydrostatic and/or inflammatory causes (a distinction that can hardly be realized using the standard physical measurement methods). Further handicaps in the evaluation of medications, which are mostly of a phytotherapeutic origin, result from an unsatisfactory analysis of the composition of the active substances in the preparations and also lack detailed knowledge of their metabolic pathways in the human body. Appropriate studies are particularly difficult to carry out with patients and alternative animal experimentation is excluded by the lack of a recognized animal model for CVI. In addition, the use of many venous therapeutics, endorsed for decades, is based on clinical studies that would not satisfy today’s standards, and moreover, the pathogenetic targets described in Part 1 of this publication have, to date, been largely ignored. Almost exclusively, purely physical parameters are at the forefront as the goal of current therapy (eg, venous pressure, leg circumference, “leg heaviness”), consistent with the classical concept that these are passive consequences of gravity. Unfortunately, therapeutic efforts frequently concentrate on cosmetic effects, neglect the causal pathogenetic mechanisms, and are often started too late. Moreover, clinical proof-of-effectiveness studies have rarely included the determination of blood parameters, such as the concentration of certain inflammatory mediators. The latter have been employed successfully in modern laboratory studies on the pathogenesis of CVI to document its inflammatory nature.11,51
Therefore, in view of all the foregoing information, the great heterogeneity of pharmacological study results is not surprising. To improve this situation, Perrin and Ramelet have suggested guidelines for the further development of appropriate drugs.52
supplements, preparations are not subject to medical supervision and assessment, and even the medicinal products are not licensed uniformly: Doxium®, for
instance, is approved in Spain only for the treatment of eye capillary fragility, whilst in other countries it is also approved for the symptomatic therapy of CVI.
* Registered as Ardium®, Alvenor®, Arvenum® 500, Capiven®, MPFF at a dose of 500 mg, Detralex®, Elatec®, Flebotropin®, Variton®, and Venitol®.
Table I: Medication for therapy of chronic venous disease (adapted from ref 67 with permission)
Questionable venous drugs The spectrum of venoactive substances is wide and comprised of different substances (Table I). The site of action of several drugs, within the framework of accepted pathogenesis models, is unclear and the clinical evidence for a therapeutic success must be frequently considered with reservations.52-54 For example, α-benzopyrone coumarin, contained in specially prepared plant extracts, is now rarely employed because of its now known hepatotoxicity and carcinogenicity.55 The evidence for the effectiveness of various synthetic venous therapeutics is also dubious. Positive clinical findings for calcium dobesilate, for instance, were reported in one study,56 while another multicenter and double-blind study found no useful effect whatsoever.57 In addition, literature57 provides no evidence for a positive effect of benzarone in the treatment of CVI, and there is no convincing evidence for naftazone effectiveness. The one positive report for the latter substance stems from a study in 1997 that does not meet today’s standards.57 The use of triterpenglycosides, (A)escin, extracted from the horse chestnut, was initially reported to be beneficial and widely prescribed,58 but is now regarded by the same authors with more reservation.59
To protect the venular endothelial barrier, a drug should inhibit the metabolic cooperation between platelets and polymorphonuclear leukocytes (PMN).60 A potential intervention for a venoactive drug would be to inhibit the intercellular clefts in the venular endothelium from opening, although this still requires a detailed biochemical investigation. Preventing the cleft opening in situ would, in any case, avoid the collapse of the venular barrier, and consequently, prevent the prompt activation of the pericytes, plasma-extravasation, and the rapid and massive coagulation and inflammatory initiation. Such specific protection of the venular barrier would appear to be a promising approach for the prevention of thrombosis and would not increase the risk of hemorrhage (see below). Protection against reactive oxygen species from activated leukocytes, a socalled antioxidant therapy, is a further desirable action of a potent venoactive drug.61
Compounds that combine all these potential actions are members of the large class of flavonoids.62 There is increasing evidence that some of these substances have a broad anti-inflammatory effect.60,62-65 Quercetin glucuronide, the main flavonoid component of the extensively studied red vine leaf extract, stabilizes specific endothelial barrier functions of the human venular endothelium at submicromolar concentrations, even in the presence of activated platelets and PMN.60,66,67 Quercetin also interferes with the proinflammatory signaling of thrombin, which results in the inhibition of adenine nucleotide secretion from activated platelets and decreased PMN function.68 Flavonoid compounds are effective platelet inhibitors, can reduce blood pressure, and restore endothelial dysfunction characterized both by a loss of the vasorelaxant effect and by reduced bioavailability of endothelial nitric oxide (NO) in hypertensive animals.69 In addition, quercetin improves vessel function by inducing endothelial NO-synthetase activity via phosphorylation of an AMP-dependent phosphokinase.70
Systemic administration of certain flavonoids has recently been recommended for the treatment of sepsis in the context of intensive care.71 This acute, life-threatening disease is characterized by generalized platelet and granulocyte activation in the entire circulation and complete breakdown of the venular barrier in numerous organs that can lead to multiple organ failure and death. Such severe pathological reactions should also be inhibited within the framework of an improved conservation and revitalization of explanted organs intended for transplant. In accordance with the central involvement of venules and their pericytes in the initiation of thrombotic events, presented in Part I, the histological diagnosis of selective venular occlusion,72 venular thrombosis,73 or venulitis74 is already regarded as the earliest reliable sign of impending organ rejection.60 Our observations on an isolated, working, blood-perfused porcine heart (xenografts in a pig-toman model of heart transplantation) show that it is extremely important to specifically prevent the venular barrier function breakdown and the resultant formation and spreading of thrombi into the venous limb of the microcirculatory system during explantation.75 Cardiac function in these experiments was significantly improved in the presence of quercetin glucuronide. Subsequent specific microscopic examination of the ventricular myocardium of such preparations showed no evidence of venular thrombosis, venulitis, or excessive accumulation of leucocytes in the myocardium.
Use in cases of mild-to-moderate chronic insufficiency (CEAP class 2 to 4)
The above-mentioned effects of quercetin glucuronides account for many aspects of their therapeutic effectiveness, as has been repeatedly demonstrated in double-blind placebo-controlled studies.76,77
In addition, a synthetically hydroxyethylated mixture of derivatives of rutin, a flavonoid extracted from plants (Table 1), has been shown to reduce leg edema and improve hemodynamics.78-80 A meta-analysis has also shown that these flavonoid preparations reduce CVI symptoms,81 and in a more recent Cochrane review, it is stated that Rutoside appears to help relieve the symptoms of varicose veins in late pregnancy.82
The clinical effectiveness of a micronized purified flavonoid fraction (MPFF) preparation has been tested frequently with respect to CVI.83-85 Data from a large (5000 patients) multicenter study are especially convincing.86 One consistent finding in clinical reports is the reduction in subjective complaints.87 This fact may be explained by a reduction in capillary leakage, which stimulates nocireceptor fibers in the tissue.
Use in chronic venous leg ulcers (CEAP class 5 to 6)
For patients with chronic venous ulcers, the most frequently tested agent is MPFF.88 A meta-analysis summarizes the data from investigations on over 700 patients.89 Corresponding clinical studies with smaller patient numbers treated with (a)escin or oxerutin failed to show faster healing or reduced recurrence of venous leg ulcers.90,91
NEW ASPECTS IN THE PHARMACOLOGICAL TREATMENT AND PREVENTION OF THROMBOSIS
As described in Part I, with respect to microvessel wall and vascular intima functional classifications, (in particular the proinflammatory and procoagulatory roles of the pericytes) highly interesting changes are looming with relation to drug treatment and prophylaxis of thrombosis.
For almost 70 years, heparins and vitamin K antagonists (VKA) have been employed as complementary anticoagulants for prevention and treatment of thrombosis. The introduction of low-molecularweight heparin (LMWH) was a big step forward with respect to both prophylaxis and treatment of DVT. Numerous studies have shown that LMWH is superior to unfractionated heparin and may even be considered for long-term treatment because it is safer than vitamin K antagonists.92 LMWH may also have more favorable effects regarding the prevention of a postthrombotic syndrome. The small LMWH molecules may diffuse more readily into the vein wall and interfere with freshly formed thrombin at the surface of procoagulant pericytes, which could explain their superior effect when compared with conventional heparin. Thrombus resolution and vein wall remodeling have also been shown to be positively influenced by LMWH.93,94
DVT restitution–treatment concentrates on clot removal by mechanical means (thrombectomy; catheterdirected thrombolysis [CDT]), but does not consider the proinflammatory and prothrombotic potential of the vein wall (see Part I). This explains the rather disappointing results with respect to prevention of the postthrombotic syndrome even after a successful CDT. This led to the most recent ACCP guideline recommending anticoagulant therapy in preference to CDT in patients with acute proximal DVT.95
If thrombolytic agents dissolved in large volumes are injected into a vein of the thrombotic leg under regional circulatory arrest, the resulting high intravenous pressure (Biers block [retrograde intravenous pressure infusion]), will promote infiltration of large fibrinolytic molecules into the thrombus, and also via the numerous venule clefts of the vasa venarum system into the interstitial space of the vein wall. This procedure, in combination with subsequent thrombectomy, may achieve better results than conventional CDT, especially in distal vein involvement.96
Vitamin K antagonists (VKA) have not changed pharmaceutically for years, although treatment surveillance has been improved by the introduction of the International Normalized Ratio (INR) and selfmonitoring/ management. Recently, however, new oral anticoagulants have been authorized. These arouse high expectations, both in prescribers and patients, since they obviate the burdensome control investigations accompanying VKA treatment, without any loss of efficiency or safety. The major difference between VKA and new anticoagulants is the highly specific action of the latter against thrombin (dabigatran etexilat, PradaxaR) or factor Xa (rivaroxaban and other “xabanes” currently under development). The half-life of these new substances is much shorter than the usual VKAs (eg, phenprocoumon, warfarin), so that “anticoagulation bridging” before surgery becomes unnecessary. The therapeutic window of the specific anticoagulants is wider, meaning that they can be administered at fixeddoses. Clinical studies have claimed that, with respect to prophylaxis of thrombosis, thromboembolism, and atrial fibrillation, these substances are as effective as conventional VKAs. Certain restrictions may become apparent with respect to gastrointestinal side effects, metabolism, renal excretion, and other routes of elimination; specific antidotes are not yet available for these effects. In short, considerable clinical experience will still be necessary before the new anticoagulants will finally replace VKAs.97
The pharmaceutical and therapeutic developments (briefly addressed above) completely ignore the recently recognized importance of hemostasis on the venular endothelial barrier, and behind it, the procoagulant pericytes, as presented in Part I. In essence, all modern anticoagulatory therapies aim to specifically inhibit already activated coagulation factors, such as factors Xa or IIa (thrombin), formed in the middle or at the end of the coagulation cascade. However, on the basis of our proposed functional model, it would be more rational to interfere with the initiation of the extrinsic coagulation pathway by blocking the tissue factor/VIIa complex on the plasmalemma and in the extracellular matrix of the pericytes. This would attenuate the accumulation of free factor Xa or thrombin in the plasma, and a physiological thrombin concentration below 0.1 nM could be established and maintained via the intrinsic coagulation pathway. The resulting constant low rate of physiological fibrin synthesis in the blood stream would then foster necessary capillary tightness (therefore reducing the risk of hemorrhage). Simultaneously, the constant activation of protein C mediated by the endothelial thrombomodulin/thrombin complex at low thrombin concentrations (see Part I, Figure 4) would continuously prevent this “physiological intrinsic fibrin production” from overshooting. Clinically employable pharmaceuticals for direct prevention of plasma factor X activation, by the pericytes cell membrane tissue factor (see Part I, Figure 4), are not yet available. However, as discussed above, certain flavonoids stabilize the integrity of the venular endothelial barrier and probably contribute to the physiological antithrombogenicity of the healthy intima, since they prophylactically prevent direct contact between pericytes and plasma coagulation zymogens. Moreover, recent studies indicate that naturally occurring flavonoids can inhibit tissue factor,98 particularly when oligopolysulfated moieties are included in the typical ring structure.98,99 In short, these substances would appear to have remarkable potential to become new, effective, and safe agents for a prophylactic anticoagulant therapy.
Flavonoid-induced sealing of the venular endothelial barrier implies possible prophylactic use, not only with respect to preventing initiation of the coagulation process in the adventitia (more precisely: at the plasmalemma and in the extracellular matrix of abundant microvascular pericytes), but also with respect to prevention of platelet activation since thrombin is the most potent physiological activator of platelet aggregation.100 Indeed, antiplatelet drugs have also been rediscovered as promising agents with respect to early interaction at the beginning of a thrombotic process in the venous system. Several recent recommendations suggest their use for preventing arterial and venous thrombosis.101,102
REFERENCES
1. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-98.
2. Partsch H, Winiger J, Lun B. Compression stockings reduce occupational leg swelling. Dermatol Surg. 2004;30:737-743.
3. van Geest AJ, Veraart JC, Nelemans P, Neumann HA. The effect of medical elastic compression stockings with different slope values on edema. Measurements underneath three different types of stockings. Dermatol Surg. 2000;26:244-247.
4. Iwama H, Furuta S, Ohmizo H. Graduated compression stocking manages to prevent economy class syndrome. Am J Emerg Med. 2002;20:378-380.
5. Partsch H, Damstra RJ, Mosti G. Dose finding for an optimal compression pressure to reduce chronic edema of the extremities. Int Angiol. 2011;30:527-533.
6. Bollinger A and Fagrell B. Clinical Capillaroscopy. A guide to its use in clinical research and practise. Hogrefe & Huber Publishers, Toronto, Lewiston (NY), Bern, Gottingen, Stuttgart, 1990.
7. Partsch H, Mosti G, Mosti F. Narrowing of leg veins under compression demonstrated by magnetic resonance imaging (MRI). Int Angiol. 2010;29:408-410.
8. Partsch B, Partsch H. Calf compression pressure required to achieve venous closure from supine to standing positions. J Vasc Surg. 2005;42:734-738.
9. Partsch H, Menzinger G, Mostbeck A. Inelastic leg compression is more effective to reduce deep venous refluxes than elastic bandages. Dermatol Surg. 1999;25:695-700.
10. Mosti G, Mattaliano V, Partsch H. Inelastic compression increases venous ejection fraction more than elastic bandages in patients with superficial venous reflux. Phlebology. 2008;23:287-294.
11. Junger M, Steins A, Hahn M, Hafner HM. Microcirculatory dysfunction in chronic venous insufficiency (CVI). Microcirculation. 2000;7:S3-S12.
12. Partsch H. [Improving the venous pumping function in chronic venous insufficiency by compression as dependent on pressure and material]. Vasa. 1984;13:58-64.
13. Partsch B, Mayer W, Partsch H. Improvement of ambulatory venous hypertension by narrowing of the femo- ral vein in congenital absence of venous valves. Phlebology. 1992;7:101- 104.
14. Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49:1013- 1020.
15. Altintas AA, Gehl B, Aust MC, Meyer-Marcotty M, Altintas MA. Die Wirkung der Kompressionstherapie auf die lokale Mikrozirkulation und Histomorphologie beim Ulcus cruris venosum. Phlebologie. 2011;40:9-14.
16. Herouy Y, Kahle B, Idzko M, Eberth I, Norgauer J, Pannier F, et al. Tight junctions and compression therapy in chronic venous insufficiency. Int J Mol Med. 2006;18:215-29.
17. Mosti G, Iabichella ML, Partsch H. Compression therapy in mixed ulcers increases venous output and arterial perfusion. J Vasc Surg. 2012;55:122- 128.
18. Mayrovitz HN, Macdonald JM. Medical compression: effects on pulsatile leg blood flow. Int Angiol. 2010;29:436-441.
19. Chen AH, Frangos SG, Kilaru S, Sumpio BE. Intermittent pneumatic compression devices – physiological mechanisms of action. Eur J Vasc Endovasc Surg. 2001;21:383-392.
20. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S-47S.
21. Partsch H, Kahn P. Venose Stromungsbeschleunigung in Bein und Becken durch “AntiThrombosestrumpfe.” Klinikarzt. 1982;11:609-615.
22. Downing LJ, Strieter RM, Kadell AM, et al. Neutrophils are the initial cell type identified in deep venous thrombosis induced vein wall inflammation. ASAIO J. 1996;42:M677-M682.
23. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835- 1846.
24. Partsch H, Lofferer O, Mostbeck A. Diagnosis of established deep-vein thrombosis in the leg using 131-I fibrinogen. Angiology. 1974;25:719- 728.
25. Froehlich JB, Prince MR, Greenfield LJ, Downing LJ, Shah NL, Wakefield TW. “Bull’s-eye” sign on gadoliniumenhanced magnetic resonance venography determines thrombus presence and age: a preliminary study. J Vasc Surg. 1997;26:809-816.
26. Rondina MT, Lam UT, Pendleton RC, et al.18 F-FDG PET in the evaluation of acuity of deep vein thrombosis. Clin Nucl Med. 2012;37:1139-1145.
27. Ogawa T, Hoshino S, Midorikawa H, Sato K. Intermittent pneumatic compression of the foot and calf improves the outcome of catheterdirected thrombolysis using low-dose urokinase in patients with acute proximal venous thrombosis of the leg. J Vasc Surg. 2005;42:940-944.
28. Mostbeck A, Partsch H, Lofferer O. [Untersuchungen zur Haufigkeit von Lungenembolien bei tiefer Beinund Beckenvenenthrombose]. Folia Angiologica. 1975;23:243-248.
29. Partsch H, Kechavarz B, Kohn H, Mostbeck A. The effect of mobilisation of patients during treatment of thromboembolic disorders with lowmolecular- weight heparin. Int Angiol. 1997;16:189-192.
30. Cohen JM, Akl EA, Kahn SR. Pharmacologic and compression therapies for postthrombotic syndrome: a systematic review of randomized controlled trials. Chest. 2012;141:308-320.
31. Deatrick KB, Luke CE, Elfline MA, Sood V, Baldwin J, Upchurch GR Jr, et al. The effect of matrix metalloproteinase 2 and matrix metalloproteinase 2/9 deletion in experimental post-thrombotic vein wall remodeling. J Vasc Surg. 2013;58:1375-1384.
32. Bouman AC, Smits JJ, Ten Cate H, Ten Cate-Hoek AJ. Markers of coagulation, fibrinolysis and inflammation in relation to the post thrombotic syndrome. J Thromb Haemost. 2012;10:1532-1538.
33. de Wolf MA, Wittens CH, Kahn SR. Incidence and risk factors of the postthrombotic syndrome. Phlebology. 2012;27:85-94.
34. Partsch H, Blattler W. Compression and walking versus bed rest in the treatment of proximal deep venous thrombosis with low molecular weight heparin. J Vasc Surg. 2000;32:861-869.
35. Partsch H, Kaulich M, Mayer W. Immediate mobilisation in acute vein thrombosis reduces post-thrombotic syndrome. Int Angiol. 2004;23:206- 212.
36. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2013;doi:10.1016/S0140- 6736(13)61902-9.
37. Brautigam P, Foldi E, Schaiper I, Krause T, Vanscheidt W, Moser E. Analysis of lymphatic drainage in various forms of leg edema using two compartment lymphoscintigraphy. Lymphology. 1998;31:43-55.
38. Partsch H, Mostbeck A. [Involvement of the lymphatic system in postthrombotic syndrome]. Wien Med Wochenschr. 1994;144:210-213.
39. Haid H, Lofferer O, Mostbeck A, Partsch H. Die Lymphkinetik beim postthrombotischen Syndrom unter Kompressionsverbanden. Med Klin. 1968;63:754-757.
40. Comerota AJ. Intermittent pneumatic compression: physiologic and clinical basis to improve management of venous leg ulcers. J Vasc Surg. 2011;53:1121-1129.
41. Oklu R, Habito R, Mayr M, Deipolyi AR, Albadawi H, Hesketh R, et al. Pathogenesis of varicose veins. J Vasc Interv Radiol. 2012;23:33-39.
42. Obermayer A, Garzon K. Identifying the source of superficial reflux in venous leg ulcers using duplex ultrasound. J Vasc Surg. 2010;52:1255- 1261.
43. Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079-1087.
44. van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
45. Labropoulos N, Bhatti A, Leon L, Borge M, Rodriguez H, Kalman P. Neovascularization after great saphenous vein ablation. Eur J Vasc Endovasc Surg. 2006;31:219-222.
46. Nyamekye I, Shephard NA, Davies B, Heather BP, Earnshaw JJ. Clinicopathological evidence that neovascularisation is a cause of recurrent varicose veins. Eur J Vasc Endovasc Surg. 1998;15:412-415.
47. Ivo CS, Ivo MB, Salles PG, Rosario RC, Nunes TA. Effect of the period of extrinsic mechanical compression following sclerotherapy in veins in rabbit ears. Acta Cir Bras. 2011;26:165- 173.
48. Weiss DR, Juchem G, Eblenkamp M, Kemkes BM, Gansera B, Geier M, et al. Search for optimized conditions for sealing and storage of bypass vessels: influence of preservation solution and filling pressure on the degree of endothelialization. Int J Clin Exp Med. 2010;3:10-27.
49. Weiss DR, Juchem G, Kemkes BM, Gansera B, Nees S. Extensive deendothelialization and thrombogenicity in routinely prepared vein grafts for coronary bypass operations: facts and remedy. Int J Clin Exp Med. 2009;2:95-113.
50. Esperester A, Schutt T, Ottillinger B. [Drugs in chronic venous insufficiency—the challenge of demonstrating clinical efficacy]. Medizinische Monatsschrift fur Pharmazeuten. 2013;36:44-51.
51. Ojdana D, Safiejko K, Lipska A, Sacha P, Wieczorek P, Radziwon P, et al. The inflammatory reaction during chronic venous disease of lower limbs. Folia Histochem Cytobiol. 2009;47:185-9.
52. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41:117-125.
53. Wollina U, Abdel-Naser MB, Mani R. A review of the microcirculation in skin in patients with chronic venous insufficiency: the problem and the evidence available for therapeutic options. Int J Low Extrem Wounds. 2006;5:169-180.
54. Word R. Medical and surgical therapy for advanced chronic venous insufficiency. Surg Clin North Am. 2010;90:1195-1214.
55. Abraham K, Wohrlin F, Lindtner O, Heinemeyer G, Lampen A. Toxicology and risk assessment of coumarin: focus on human data. Mol Nutr Food Res. 2010;54:228-239.
56. Widmer L, Biland L, Barras JP. Doxium 500 in chronic venous insufficiency: a double-blind placebo controlled multicentre study. Int Angiol. 1990;9:105-110.
57. Martinez MJ, Bonfill X, Moreno RM, Vargas E, Capella D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2005:CD003229.
58. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2006:CD003230.
59. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2012;11:CD003230.
60. Juchem G, Weiss DR, Knott M, Senftl A, Forch S, Fischlein T, et al. Regulation of coronary venular barrier function by blood borne inflammatory mediators and pharmacological tools: insights from novel microvascular wall models. Am J Physiol Heart Circ Physiol. 2012;302:H567-H581.
61. Firuzi O, Miri R, Tavakkoli M, Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18:3871-3888.
62. Manthey JA. Biological properties of flavonoids pertaining to inflammation. Microcirculation. 2000;7:S29-S34.
63. Gonzalez-Gallego J, Garcia-Mediavilla MV, Sanchez-Campos S, Tunon MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104:S15-S27.
64. Serafini M, Peluso I, Raguzzini A. Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 2010;69:273-8.
65. Magrone T, Jirillo E. Polyphenols from red wine are potent modulators of innate and adaptive immune responsiveness. Proc Nutr Soc. 2010;69:279-285.
66. Nees S, Weiss DR, Juchem G. “Schwere Beine” verstehen, Neues zur Pathogenese der chronischen Veneninsuffizienz. Deutsche ApothekerZeitung. 2013;153:3186-3190.
67. Nees S, Weiss DR, Juchem G. Pharmakologische Therapie der CVI. Deutsche ApothekerZeitung. 2013;153:3192-196.
68. Kaneider NC, Mosheimer B, Reinisch N, Patsch JR, Wiedermann CJ. Inhibition of thrombin-induced signaling by resveratrol and quercetin: effects on adenosine nucleotide metabolism in endothelial cells and platelet-neutrophil interactions. Thromb Res. 2004;114:185-194.
69. Larson A, Witman MA, Guo Y, Ives S, Richardson RS, Bruno RS, et al. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: nitric oxide. Nutr Res. 2012;32:557-564.
70. Shen Y, Croft KD, Hodgson JM, Kyle R, Lee IL, Wang Y, et al. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem Pharmacol. 2012;84:1036-1044.
71. Shapiro H, Lev S, Cohen J, Singer P. Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition. 2009;25:981-997.
72. Faioni EM, Mannucci PM. Venocclusive disease of the liver after bone marrow transplantation: the role of hemostasis. Leuk Lymphoma. 1997;25:233-245.
73. Rose AG, Cooper DK. Venular thrombosis is the key event in the pathogenesis of antibody-mediated cardiac rejection. Xenotransplantation. 2000;7:31-41.
74. Hubscher SG. Central perivenulitis: a common and potentially important finding in late posttransplant liver biopsies. Liver Transpl. 2008;14:596- 600.
75. Juchem G, Bauer A, Postrach J, Reichart B, Nees S. Quercetin glucuronide preserves cardiac function in porcine hearts during perfusion with human whole blood by inhibiting platelet/neutrophil activation and preserving venular barrier function. The FASEB Journal. 2012;26:857.4.
76. Rabe E, Stucker M, Esperester A, Schafer E, Ottillinger B. Efficacy and tolerability of a red-vine-leaf extract in patients suffering from chronic venous insufficiency–results of a double-blind placebo-controlled study. Eur J Vasc Endovasc Surg. 2011;41:540-57.
77. Kiesewetter H, Koscielny J, Kalus U, Vix JM, Peil H, Petrini O, et al. Efficacy of orally administered extract of red vine leaf AS 195 (folia vitis viniferae) in chronic venous insufficiency (stages I-II). A randomized, doubleblind, placebo-controlled trial. Arzneimittelforschung. 2000;50:109-117.
78. Grossmann K. Comparison of the efficacy of a combined therapy of compression stockings and Venoruton vs. compression stockings and placebo in patients with CVI. Phlebology / Venous Forum of the Royal Society of Medicine. 1997;26:105-110.
79. Petruzzellis V, Troccoli T, Candiani C, Guarisco R, Lospalluti M, Belcaro G, et al. Oxerutins(Venoruton): efficacyinchronic venous insufficiency-a double-blind, randomized, controlled study. Angiology. 2002;53:257-263.
80. Unkauf M, Rehn D, Klinger J, de la Motte S, Grossmann K. Investigation of the efficacy of oxerutins compared to placebo in patients with chronic venous insufficiency treated with compression stockings. Arzneimittel- Forschung. 1996;46:478-482.
81. Poynard T, Valterio C. Metaanalysis of hydroxyethylrutosides in the treatment of chronic venous insufficiency. VASA Zeitschrift fur Gefasskrankheiten. 1994;23:244-50.
82. Bamigboye AA, Smyth R. Interventions for varicose veins and leg oedema in pregnancy. Cochrane Database Syst Rev. 2007:CD001066.
83. Blume IL, Chamvallins M. Quantification of oedema using the volunteer technique: therapeutic application of MPFF at a dose of 500 mg in CVI. Phlebology / Venous Forum of the Royal Society of Medicine. 1992;2:37-40.
84. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G.. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology. 1997;48:45-9.
85. Ting AC, Cheng SW, Wu LL, Cheung GC. Clinical and hemodynamic outcomes in patients with chronic venous insufficiency after oral micronized flavonoid therapy. Vasc Surg. 2001;35:443-447.
86. Jantet G. RELIEF study: first consolidated European data. Reflux assEssment and quaLity of lIfe improvement with micronized Flavonoids. Angiology. 2000;51:31-37.
87. Lyseng-Williamson A, Perry CM. Micronised purified flavonoid fraction. A review of its use in chronic venous insufficiency, venous ulcers and haemorrhoids. Drugs. 2003;63:71-100.
88. Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7:303-308.
89. Coleridge-Smith P. MPFF at a dose of 500 mg and venous leg ulcer: new results from a meta-analysis. Angiology. 2005;56:S33-S39.
90. Wright DD, Franks PJ, Blair SD, Backhouse CM, Moffatt C, McCollum CN. Oxerutins in the prevention of recurrence in chronic venous ulceration: randomized controlled trial. Brit J Surg. 1991;78:1269-1270.
91. Leach MJ, Pincombe J, Foster G. Clinical efficacy of horsechestnut seed extract in the treatment of venous ulceration. J Wound Care. 2006;15:159- 167.
92. Andras A, Sala Tenna A, Crawford F. Vitamin K antagonists or lowmolecular- weight heparin for the long term treatment of symptomatic venous thromboembolism. Cochrane Database Syst Rev. 2012:CD002001.
93. Moaveni DK, Lynch EM, Luke C, Sood V, Upchurch GR, Wakefield TW, et al. Vein wall re-endothelialization after deep vein thrombosis is improved with low-molecular-weight heparin. J Vasc Surg. 2008;47:616-624.
94. Sood V, Luke C, Miller E, Mitsuya M, Upchurch GR Jr, Wakefield TW, et al. Vein wall remodeling after deep vein thrombosis: differential effects of low molecular weight heparin and doxycycline. Ann Vasc Surg. 2010;24:233-241.
95. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S-94S.
96. Blattler W, Heller G, Largiader J, Savolainen H, Gloor B, Schmidli J. Combined regional thrombolysis and surgical thrombectomy for treatment of iliofemoral vein thrombosis. J Vasc Surg. 2004;40:620-625.
97. Baumann Kreuziger LM, Morton CT, Dries DJ. New anticoagulants: A concise review. J Trauma Acute Care Surg. 2012;73:983-992.
98. Lee MH, Son YK, Han YN. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch Pharm Res. 2002;25:842-850.
99. Correia-da-Silva M, Sousa E, Duarte B, Marques F, Carvalho F, Cunha- Ribeiro LM, et al. Flavonoids with an oligopolysulfated moiety: a new class of anticoagulant agents. J Med Chem. 2011;54:95-106.
100. Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381-1389.
101. Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e89S-119S.
102. Warkentin TE. Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med. 2012;367:2039-2041.