Conservative treatment of chronic venous disease: the Italian experience
Department of Clinical Sciences and
Community Health, University of Milan,
Milan, Italy.
ABSTRACT
Chronic venous disease (CVD) is a highly prevalent clinical condition with substantial epidemiological implications and socioeconomic repercussions. CVD is a worldwide problem and does not only affect patients in Western countries. The consequences of its high prevalence, diagnosis and therapy costs, significant worker absenteeism, and its impact on patients’ quality of life are well known. Pharmacotherapy for CVD, solely represented by venoactive drugs, has seen great developments over the last 40 years. Venoactive drugs are widely used in the symptomatic treatment of CVD together with compression therapy in order to relieve patient suffering. The clinical efficacy of venoactive drugs on symptoms (sensations of heaviness, leg pain, paresthesia, sensations of warmth and burning, swelling, night cramps, etc) has long been confirmed by both open and end-controlled studies. We carried out a joint analysis of the management and costs of CVD in Italy and found that pharmacotherapy is very useful in both. Double blind randomized trials have used micronized purified flavonoid fraction, rutosides, escin, anthocyanosides, and synthetic calcium dobesilate in the treatment of CVD. In the following state-of-the-art review article, we have used evidence-based medicine methods to review publications found in the medical literature. Particular consideration was given to the evidence obtained in meta-analyses, guidelines, and consensus statements. Currently, the evidence for pharmacological agents such as venoactive drugs in the treatment of CVD suggests their wide use for the resorption of venous edema and the relief of venous symptoms associated with all classes of the CEAP classification.
Chronic venous disease (CVD), defined as morphological and functional abnormalities of the venous system of long duration, manifested by symptoms or signs or both indicating the need for investigation and/or care,1 has a documented socioeconomic impact, affecting 50% to 85% of Western populations, and consuming 2% to 3% or more of community health care budgets.1
For a long time and until the early 1990s, the definition of CVD had been limited solely to the presence of varicose veins and/or ulcers, managed with a single treatment, ie, surgical crossectomy/stripping of the diseased vein, and the management of venous ulcers. This is reflected in the epidemiological studies published at the time, which focused on these two conditions.2 However, not all specialists were in favor of systematic resection of the diseased veins in patients with varicose veins, and some renowned specialists such as Felix Jaeger and Edmondo Malan recommended varicose vein ablation only after careful examination and investigation. Venous pain and symptoms usually associated with varicose veins and ulcers were not taken into account.
It was only in February 1994, at the Sixth Annual Meeting of the American Venous Forum (AVF), that an international ad-hoc committee with representatives from Australia, Europe, and the USA, proposed a more comprehensive view of phlebology and its associated conditions. This commission produced a consensus statement for the classification of all stages of CVD referred to by the acronym “CEAP” and based on the clinical manifestations (C), etiological factors (E), anatomical distribution (A), and pathophysiological findings (P) of venous disease. The goal was to provide an objective, comprehensive classification that could be used worldwide, first in scientific research, then in specialist clinical practice, and lastly in general medical practice.3 According to the CEAP classification, the clinical signs in the affected legs are categorized into seven classes designated C0 to C6. Leg symptoms associated with CVD include tingling, aching, burning, pain, muscle cramps, sensation of swelling, sensations of throbbing or heaviness, itching skin, restless legs, leg tiredness and/ or fatigue.1 Although not pathognomonic, these signs and symptoms may be suggestive of CVD, particularly if they are exacerbated by heat or dependency during the course of the day and relieved with leg rest and/or elevation.1 Limbs categorized in any clinical class may be symptomatic (S) or asymptomatic (A). The latest revision of the CEAP classification in 2004 included a new descriptor for the E, A, and P sections of the CEAP classification, when no anomaly is found in the etiology, anatomy, or pathophysiology of the disease.4 This new descriptor introduced new categories such as C0s (“symptoms only”), En, An, Pn (“no etiology, no location, no pathophysiology identified”), reflecting those subjects complaining of leg symptoms before the occurrence of any sign, reflux, or even obstruction. The latter is usually difficult to identify. C0s patients are frequently encountered in primary care practice. In the CEAP classification, the old term “varicose” has now been replaced by more complex definitions whereby “chronic venous disorders” is the term used for the description of the full spectrum of signs and symptoms associated with classes C0s to C6, the term “chronic venous disease” encompasses the CEAP clinical classes C1 to C6, and the term “chronic venous insufficiency” (CVI) is generally restricted to disease of greater severity (ie, classes C3 to C6).1
All stages of the disease require medical attention, generally from the end of the first stages, when symptoms and signs are apparent, but also right from the onset of the disease when patients present with simple cosmetic imperfections, before any possible serious and disabling adverse effects such as ulceration, or potentially lethal events such as episodes of venous thromboembolism occur.
Given the broad spectrum of conditions that CVD encompasses and the high incidence of the first stages of the disease (before the occurrence of varicose veins), it is clear that CVD is of primary interest for conservative management—as evidenced by recent guidelines (Figure 1).5‑7 Despite this, the use of conservative treatment will likely regress, since, for the sake of necessary savings, health care policies have contributed to shift the treatment of CVD from the expertise of venous specialists to generic and cosmetic approaches, the so-called “alternative therapies.”
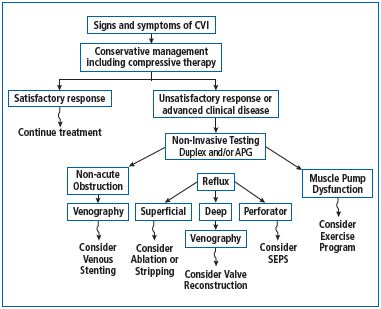
Figure 1. Management of chronic venous disease: clinical
algorithm (adapted from reference 7: Eberhardt RT, et al).
Since 2000, most epidemiological studies on CVD have used the CEAP classification. Among them, the recent international epidemiological program named “Vein Consult Program” (VCP) initiated by the Union Internationale de Phlébologie has provided reliable results on the global epidemiology of CVD in 20 countries and 5 continents and shows that CVD affects a significant part of the world’s population. The prevalence of CVD was found to be 64%, when considering patients classified between the C1 and the C6 class.8 This prevalence, which may seem high, is in fact comparable to and even lower than the corresponding figures from other studies carried out with the use of the CEAP: 90% in the Bonn Vein Study (Germany),9 49% in Poland,10 77% in the Italian study by Chiesa et al11 that included 24 Italian towns, 83% in Bulgaria,12 and 71% in the USA.13
The prevalence of CVD in the VCP even rose to 84% when C0s subjects were included, with 20% of subjects being in the C0s class, 22% in the C1 class (telangiectasias), 18% in the C2 class (varicose veins), 15% in the C3 class (edema), and 9% in the C4 to C6 classes (complications of CVD).8 Healed and active ulcers were equally present in men and women in the VCP population. This is in line with the results of the Bonn Vein Study.9 In most epidemiological studies, active ulceration in the lower limbs (C6) has been reported in about 0.3% of the adult population of Western countries. However, the number of these ulcers, including those that had healed (class C5: with signs of ulceration of the skin), amounts to at least 1% of the population. The findings of the VCP were close to these figures: 1.4% of subjects were in C5 and 0.7% in C6. These findings suggest a worldwide progression of CVD, which affects not only Western countries, as is too often believed, but all continents.
Globally, the quality of life was worse in all subjects diagnosed as CVD patients in the VCP study, and not only for those with advanced stages of the disease. This is in line with a number of previous studies.14,15 Despite this, it is noteworthy that the diagnosis of CVD is usually underestimated, especially in the early stages and, consequently, conservative treatment—venoactive drugs and compression therapy—is under prescribed. An Italian study assessing the self-management of CVD in a selected Italian population and the pattern of prescription by selected Italian phlebologists revealed that physicians tended to suggest a treatment to more people than those already under treatment, and they also tended to recommend the same therapy as the one already used by their patients (Figure 2).14 The patients preferred conservative treatment—mainly drug therapy—over surgery or sclerotherapy. As a result, the recommendation to use compression stockings, believed to be the treatment of choice in CVD, was progressively replaced by the recommendation to use venoactive drugs (Figure 2).
Over the last 15 years, various health policy instruments have influenced the comparison of the scientific and economic aspects of CVD. In Italy, we have produced the first evidence-based guidelines in the management of chronic venous disorders in order to implement a correct approach to the diagnostic and therapeutic management of CVD.16 The Italian guidelines state that, in a patient with varicose vein disease, “the indication for surgery should be thoroughly discussed, independently of the surgical option chosen. The aim of surgery is total removal of all varicose veins, and this itself must be viewed in the context of the underlying pathology—CVD—and the troublesome problem of varicose veins recurring and new ones appearing after surgery. The main aim of treating patients with CVD is to cure or relieve the symptoms and to prevent or treat complications.” Regarding the use of drug therapy, the Italian guidelines state that “there is ample evidence in favor of a therapeutic strategy including venoactive drugs when surgery is not indicated or not feasible. Venoactive drugs are indicated for subjective symptoms related to CVD, whatever the stage of the disease (fatigue, nighttime cramps, restless legs, heaviness, tension), to reduce edema and according to a meta-analysis, as adjunctive treatment in the healing of venous leg ulcers (only adjunctive micronized purified flavonoid fraction [MPFF]* was shown to be effective in leg ulcer healing).”16
*MPFF is registered as: Alvenor®, Ardium®, Arvenum® 500, Capiven®, MPFF at a dose of 500 mg®, Detralex®, Elatec®, Flebotropin®, Variton®, Venitol®
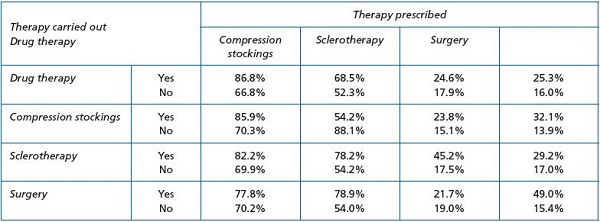
Figure 2. Correlation between recommendation by the phlebologist of a therapy and reports of this therapy having been carried out by
the respondents (from reference 14: Marone EM, et al.).
However, the problem of rising health care costs has prompted a reform in Italy, which resulted in counterproductive measures. The eimbursement of venoactive drugs in 1994, combined with the ongoing nonreimbursement of elastic stockings (despite the fact that stockings are reimbursed in all other European countries), caused a significant fall in the number of visits to family doctors and in the number of prescriptions, including those for venoactive drugs. This left the burden on patients with CVD and has had a definite negative impact on their health (Figure 3).17
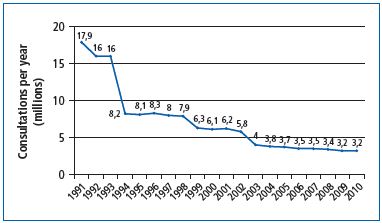
Figure 3. Number of chronic venous disease–related general
practitioner office visits/year (1991 to 2010) (from reference 17:
Allegra C.).
The question of the cost-effectiveness of venoactive drugs has been analyzed more frequently in other countries of the European Union.18 The Italian study on the benefits of the reform, partly based on data collected in the Lombardy region,17 has evidenced the impact produced by another measure of the reform of the social security system, and how it changed the direction of the treatment of CVD. Directive 119 of January 1st, 1995, by giving preference to surgical treatment over conservative therapy, has prompted an increase in the number of surgical procedures, which rose to 136 075 varicose vein ligations and stripping procedures in 2009 (according to official data). Actually, the post-reform cost redistribution from prescriptions and visits in favor of hospitalization—an aggressive form of treatment that is clearly inappropriate for certain indications—ended up increasing CVD management expenses. The World Health Organization (WHO) considers that preventing chronic diseases is a ‘vital investment’ and is deeply concerned by “the global neglect of chronic diseases.”19 The post-reform results in Italy point to the need to consider “effective and feasible interventions that may halt and turn back the growing threats of chronic diseases with minimal spending” as reported by the WHO.19
The confusion created in recent years by the dissemination of dietary supplements allegedly considered as therapies and not as health adjuvants has also contributed to the reduction of venoactive drug use. The market for venoactive drugs seems to be increasingly driven by dietary supplements. The latter, which can be bought in “parapharmacies” [drug stores selling cosmetic products and dietary supplements], accounted for 20.4% of the total market share of venoactive agents in 2004, while they amounted to nearly 28% of the total market in 2010. Sales of dietary supplements are increasing at the expense of ethical venoactive drugs, which are dispensed in pharmacies.
The sales volume and indications for use of elastic stockings for CVD, including the purchase of at least two pairs per year, authorized by either the Social Security System or the private insurance system (depending on the countries considered), based on precise therapeutic indications,20 are extremely difficult to determine. In the pharmaceutical industry in Europe (EUEOCOM), there are contrasting results. For example in France, with a population and number of patients affected by CVD similar to those in Italy, it is estimated that the market amounts to 7 million pairs of stockings per year. In Germany, the number of pairs of stockings sold for curative purposes is 3.5 million per year. However, the criteria for prescription differ between the two countries. In Germany, stockings are prescribed free of charge with a request for a precise description of the disorder. On the contrary, in France, it is considered easier to obtain a prescription for stockings. But are these stockings actually worn by patients? In Italy there is a market for stockings prescribed for curative purposes and the available data show that about 500 000 to 600 000 pairs of stockings are sold per year. It is clear that some confusion still exists in the minds of many specialists about the use of stockings for curative and preventive purposes.
Conservative therapy of CVD is based on three cardinal tenets: i) lifestyle changes and correction of functional abnormalities with physical methods, ii) pharmacological therapy, and iii) compression therapy.
Changes in lifestyle are part of the 2010 directives of the Italian Ministry of Health, which in 2011 instituted the “National platform on diet, physical activity, and smoking,” chaired by the Ministry of Health, and implemented a major program of prevention throughout the country entitled “Preserving your health: making healthy choices easier,” which aimed at limiting the main risk factors for chronic venous disorders (as well as other disorders), ie, smoking, alcohol abuse, poor diet, and lack of physical activity. Such initiatives, considered innovative in Italy and relying on the responsibility of both patients and health care officials make medical advice a vital component in the early prevention of CVD.
There are different options for the correction of functional anomalies with physical methods and evidence of their efficacy has led to their widespread implementation being recommended. This is true for hydrotherapy, an ancient therapy, which from Kneipp’s principles to modern spa therapy has now been confirmed by randomized studies as well as by phlebologists.16,21 This is also true for venous-lymphatic massage therapy,22 programs of tilt-table therapy, and physiotherapy for more advanced stages in the CEAP classification,16,23-25 and correction of posture with plantar arch support.26,27
Modern pharmacological therapy of CVD should now specifically target the primary cause of the disease. Primary CVD is the result of increased and unabated venous hypertension caused mostly by reflux through incompetent venous valves, and sometimes by primary non-postthrombotic obstruction and reflux.28 Venous hypertension is central to the alterations present in the superficial veins (less frequently in the deep veins), in capillaries, and eventually, skin changes.29 Histological and ultrastructural studies of varicose veins have found alterations in the venous wall with hypertrophic segments alternating with hypotrophic segments. Hyperplasia of the intima, with increased collagen content and smooth muscle cell (SMC) disruption, is seen in hypertrophic areas, while atrophic areas show a lower number of SMCs and reduced extracellular matrix because of degradation of the matrix by proteolytic enzymes, including metalloproteinases (MMPs).29-31 Remodeling of the venous wall probably arises from complex synergy between many factors, including alterations related to MMPs and tissue inhibitors of metalloproteinases (TIMPs), high levels of catechin and growth factors, which together promote extracellular matrix degradation.30,31
A growing body of evidence collected over the last few years shows that neutrophils, mast cells, and their interactions with the venous endothelium play an important role in initiating a specific inflammatory response leading to venous dysfunction.29 The venous wall alterations resulting from inflammatory processes contribute to dilation of the veins of the lower limbs with loss of venous tone. The role of the microcirculation in this disease process should not be overlooked.
Leukocyte and endothelium interaction is the first phase of the inflammatory process causing the remodeling process of the venous wall and the venous valves. This process is characterized by the expression of the adhesion molecules VCAM-1 and ICAM-1 on the endothelial cells and by monocyte chemotaxis. The process of adhesion consists in the leukocytes rolling over the surface of the endothelial cells with intervention of the P- and E-selectins, VCAM-1, and ICAM-1. Then, a strong bond is established between the integrins, such as VLA-4 and CD11b/CD18, on the surface of the leukocytes and the endothelial adhesion molecules, allowing entry of the leukocytes into the subendothelial layer.29
Migration of the leukocytes in the venous wall produces toxic metabolites and free radicals, which damage the venous valves and weakening the venous wall. Valvular incompetence of the superficial and perforating veins leads to increased pressure in the veins and venules in the cutaneous and subcutaneous tissue, leading to capillary damage, edema, skin changes (pigmentation), and lastly, venous ulceration.7,29
Lastly, the role of alterations in the glycoprotein providing the endothelial surface with an antiadhesive property is essential.32 The lymphatic circulation is also involved in more advanced stages of the disease process.
The purpose of this article is not to express a new viewpoint on the etiopathogenic aspects of CVD. Dedicated state-of-the-art articles have already reviewed those events in depth.7,29
Basic research, however, may have a direct impact on the pharmacological treatment of CVD, which affects a large number of subjects as seen through epidemiological data and which should be resolutely applied before any surgical intervention. Actually, pharmacological therapy should not be reserved solely for those patients–particularly the elderly population— who are ineligible or unwilling to undergo venous surgery or endovenous treatment,33 but should be considered from the earliest stages of the disease, right from the C0s class. Venoactive drugs have been shown to have the following properties. They:
– increase venous tone;
– limit stasis in the microcirculation;
– improve lymphatic drainage;
– decrease capillary hyperpermeability;
– hamper inflammation in the vein wall, venous valves, and capillaries.
Therefore, by acting on the many targets responsible for the symptoms and signs of CVD, venoactive drugs have a beneficial effect on CVD and may prevent it from getting worse.
The effects of venoactive drugs on these physiological parameters, in particular, on venous tone, venous hemodynamics, capillary permeability, and lymphatic drainage, can be evaluated experimentally or clinically, preferably with noninvasive methods.
Advanced technology processes, such as micronization, have improved the efficacy of some drugs. The most famous example is that of the micronized purified flavonoid fraction (MPFF) whose comprehensive mechanism of action can be attributed to its specific formulation, together with the micronization of its active ingredients. MPFF consists of 90% diosmin and 10% other flavonoids (hesperidin, diosmetin, linarin, and isorhoifolin). Each of the active ingredients acts synergistically with diosmin. In addition, the micronization process, which reduces active substance particles to a size of under 2 μm, increases the absorption and dose-dependent efficacy of MPFF compared with nonmicronized forms, for which an increase in dosage does not correspond to an increase in efficacy (Figure 4).34
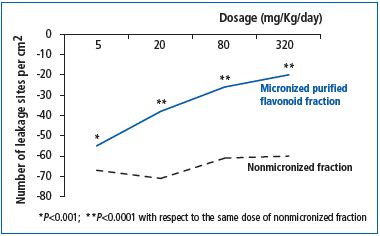
Figure 4. Dose-dependent anti-edema efficacy of micronized
purified flavonoid fraction compared with the nonmicronized
form (from reference 34: Cyrino F, et al)
Most of the experimental and randomized controlled clinical trials that have been analyzed in reviews were carried out with MPFF,34-38 rutosides,35,39-41 escin, anthocyanosides, and synthetic calcium dobesilate.5,35 Most venoactive drugs have been shown to increase venous tone by a mechanism related to the noradrenaline pathway. MPFF prolongs noradrenergic activity, thereby decreasing the metabolism of norepinephrine and prolonging its venoconstrictor effects. Hydroxyethylrutosides act by blocking the inactivation of noradrenaline, and escin and ruscus extracts exert an agonist action on venous β1-adrenergic receptors.5,35
A number of pharmacological trials have shown that venoactive drugs increase capillary resistance and reduce capillary filtration, resulting in the prevention of capillary leakage. This has been demonstrated for MPFF, rutosides, escin, ruscus extracts, proanthocyanidins, and calcium dobesilate.5
Pharmacological trials of oral pharmaceutical treatments including coumarin and its derivatives, hydroethylrutosides, calcium dobesilate, escin extracts, O-(beta-hydroxyethyl)-rutosides, and MPFF have found that such drugs may help in the treatment of lymphedema by reducing vascular permeability and protein and extracellular fluid accumulation, stimulating lymph contractility and flow, and reducing protein concentration and fibrotic induration in the tissues by stimulating proteolysis. In addition, microlymphography has shown that MPFF induces an increase in lymphatic function by improving lymph flow drainage and increasing the number of lymphatic vessels.5
Regarding inflammation in the venous valves and wall, MPFF treatment significantly attenuated the reduction in valve height in pressurized veins, and the rate of retrograde blood flow at 3 weeks was markedly reduced with MPFF compared with control. MPFF showed a trend toward a reduction in granulocyte infiltration into the valves. When compared with the control, MPFF treatment inhibited the expression of the endothelial cell adhesion molecules P-selectin and ICAM-1, reduced leukocyte infiltration, and decreased the level of apoptosis in the valves in a dose-dependent manner. These data suggest that in the rat model of venous hypertension, MPFF attenuates the alterations in valve shape and lowers the subsequent hemodynamic disturbances. A concomitant decrease in leukocyte mediated valve inflammation was observed. In a former study, MPFF was shown to decrease the expression of endothelial and leukocyte adhesion molecules (ICAM- 1, VCAM, CD11b, CD62L). None of the other available drugs have been shown to attenuate leukocyte endothelial interactions in vivo.5,29,42,43
Well-conducted clinical trials with clear inclusion criteria and evaluable end points and compliance with ethical prerequisites constitute the best instrument to evaluate the clinical effects of venoactive drugs. Any clinical trial should be randomized, possibly double blinded, with sufficient power to provide a response to a well-defined question. This is now simpler than in the past, with the introduction of the CEAP classification providing a framework to improve patient description and report response to treatment.
From the standpoint of clinical research, let us keep in mind that venoactive drugs—products of natural, seminatural or synthetic origin—belong to the class of bioflavonoids in their vast majority. Some (such as MPFF) contain a combination of active substances for increased efficacy and perform better than others in improving clinical symptoms and signs.
Venoactive drugs can be administered at all stages of CVD to decrease symptoms (feeling of heaviness, pain, paresthesias, sensations of warmth and burning, nighttime cramps, pruritus), with various grades of evidence. MPFF has been shown to exert its action at all stages of CVD and has been found to be significantly more effective than nonmicronized diosmin in reducing the principal symptoms and signs of CVD. Also, clinical effectiveness on the principal target sign and symptom observed in CVD—edema—was evident with MPFF in a study conducted on patients with lower limb edema. In only 6 weeks, MPFF reduced the amount of fluid in the lower limbs by about one liter (Figure 5).44
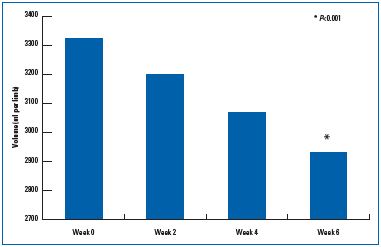
Figure 5. Anti-edema efficacy over time of the active substances
in the micronized purified flavonoid fraction (from reference 44:
Blume et al.).
Lastly, in patients with venous ulceration, MPFF reduces the time to healing and increases the number of healed ulcerations. Furthermore, another series of studies points in this direction, with the use of sulodexide in the advanced stages of CVD, especially in post thrombotic syndrome. By demonstrating the utility of treatment in patients with venous ulceration, the latest North American guidelines reported the effectiveness of MPFF and sulodexide and recommended their use.45
In addition to the clinical assessment of the beneficial effects of drugs, determination of patient quality of life (QOL) has been accorded increasing scientific attention in recent years, mainly because the question of the direct benefit of medical treatment for patients has been raised.
The use of the 20-item ChronIc Venous dIsease Questionnaire (CIVIQ-20) through the Reflux assEssment and quaLity of lIfe improvEment with micronized Flavonoids (RELIEF) study has confirmed that MPFF improves venous symptoms (pain, sensation of swelling, cramps), and reduces edema in the presence and/or absence of venous reflux.46 The CIVIQ questionnaire has been used to assess the beneficial effect of various treatments.47
The paucity of “quality” studies on many venoactive drugs in the literature is evident in recent systematic revisions. A group of experts meeting at the 13th European Conference of the Society of Clinical Hemorrheology (Siena, 2005) identified only 83 studies that deserved further consideration.48 Similar considerations emerged from the recent Cochrane reviews of horse chestnut seed extracts (HCSE),49 and of venoactive drugs as a whole.50 In the first review, only 17 of the 44 identified trials were analyzed, while in the second, 110 randomized trials were identified, but only 44 were retained.
Based on these reviews, significant and homogeneous results were found for most venoactive drugs on edema reduction, a decrease in restless legs, and improvement of trophic disorders.
Some venoactive drugs performed better than others in improving venous disorders. Considering the evidence, a higher grade recommendation (Grade A) for the treatment of symptoms and of edema was attributed only to MPFF and oxerutin,48 while only MPFF and sulodexide were recommended in the treatment of patients with a venous ulceration.35
Some mention should be made of the increased use of dietary supplements by patients with venous disease, already described above as a “commercial” phenomenon.
Most of these so-called ‘dietary supplements’ are medicinal products presented as having curative or preventive properties in humans or in animals, herbal products marketed as a food, dietary supplement, drug, or cosmetic,45 or dietary supplements, defined as physiological and “noncurative” adjuvant products. Many doubts persist on the actual effectiveness of these treatments for which clinical evidence is scarce or anecdotal, in the absence of randomized clinical trials. With the exception of currently available drugs, no clinical evidence exists on the pharmacological efficacy against CVD of any other phytotherapy or herbal products on the market.
Compression therapy is considered to be a basic treatment for CVD of the lower limbs in different CEAP classes for its effects on venous hemodynamics, hydrostatic pressure in the large superficial and deep veins, the microcirculation, coagulation and fibrinolysis, and edema reduction.
Compression of venous vessels is obtained through the application of bands or of long bandages for medium-or short-term periods; elastic stockings of various shapes and sizes can be used in various cases depending on therapeutic requirements, with a certain preference for bandaging in treatment of acute cases, and stockings for chronic cases.
In the European Union, quantification of the levels of compression depends on national standards since a community-wide reference standard has not been adopted to date.
National and international guidelines are available on proper prescription and use of compression therapy.51-54
Due to the current prevalence and spread of CVD and its effect on quality of life,55 some critical measures should be taken as the attention paid to CVD has decreased in recent years in Italy. In fact, a medico-scientific analysis of CVD should not disregard the economic, ethical, and intersocietal aspects as well as the overall impact of the disease.
From an economic standpoint, as discussed above, the dereimbursement of medicinal products for CVD and of elastic compression stockings, as well as the increase in taxation on these products, makes it more difficult for family doctors and vascular specialists to prescribe conservative therapy for the prevention of this disease.
Meanwhile, little attention is being paid by national and regional health care authorities to the increasing use of inappropriate dietary supplements for this disorder. The economic disengagement of the government and the legal ramifications of prescribing noncurative substances remain largely ignored.
Also, insufficient attention is being paid to the current inappropriate emphasis on surgery, which is debatable in the current worsening economic situation. The increasing importance given to surgery is controlled by the irrational lowering of the fee schedules in diagnosis-related groups (DRGs) and the imposition of inappropriate surgical indications (from ordinary hospital admissions to daycare surgery, to ambulatory surgery for varicose veins).
In addition, surgery itself is now the source of a “decline” in the attention given to phlebology, because of the senseless dispute involving the use of different surgical techniques and strategies, such as stripping versus the CHIVA method (ambulatory and hemodynamic treatment of venous disease), stripping versus endovascular treatments, endovenous laser therapy versus foam sclerotherapy, etc. This dispute, sometimes extraneous to the discussion of scientific methodology (comparing results presented at congresses and in specialized scientific journals), is circulated in inappropriate places (newspapers, TV, internet) creating bewilderment and confusion in the same media and in governmental authorities, as well as confusion and anxiety for patients and family doctors.
Also, among the current criticisms, we should not overlook the under evaluation of CVD, and the current fragmentation created by too many scientific societies and active “groups” in the field of phlebology.
CONCLUSIONS
Attention to the medical treatment of CVD should involve family doctors as well as vascular specialists and the other medical fields involved in CVD, in particular obstetrics-gynecology, orthopedics, etc. A commitment to lifestyle changes is required for the treatment of CVD, and this, for all the clinical classes of the CEAP classification.
Evidence-based medicine—randomized clinical trials, reviews, and meta-analyses—supports this attention, pointing to the efficacy of venoactive pharmacological therapy, together with more appropriate use of certified elastic stockings in the treatment of CVD.
Among medicinal products, MPFF is the only venoactive agent available in micronized form to have a well-known complete mechanism of action. This medicinal product and a few others have been shown to improve symptoms (such as pain) and signs (such as edema), which leads to a consistent improvement in quality of life.
The recommendations of national and international guidelines therefore suggest a wider treatment strategy for CVD along these lines.

REFERENCES
1. Eklof B, Perrin M, Delis KT, et al. Updated terminology of chronic venous disorders: The VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49:498-501.
2. The management of chronic venous disorders of the leg: An evidence-based report of an international task force. Phlebology. 1999;14(suppl 1):1-126.
3. Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic venous Disease. J Vasc Surg. 1995;21:635-645.
4. Eklof B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248- 1252.
5. Nicolaides A, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
6. Coleridge Smith PD. Drug treatment of varicose veins, venous oedema, and ulcers. In: Gloviczki P, editor. Handbook of venous disorders: guidelines of the American venous Forum. 3rd ed. London, UK: Hodder Arnold; 2009:359-365.
7. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111:2398-2409.
8. Rabe E, Guex JJ, Puskas A, et al. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult program. Int Angiol. 2012; 31:105-115.
9. Rabe E, Pannier-Fischer F, al. Bonner Venenstudie der Deutschen Gesellschaft für Phlebologie. Phlebologie. 2003;32:1-14.
10. Jawien A, Grzela T, Ochwat A. Prevalence of chronic venous insufficiency (CVI) in men and women in Poland: multicenter cross-sectional study in 40 095 patients. Phlebology. 2003;18:110-122.
11. Chiesa R, Marone EM, Limoni C, Vet al. Chronic venous insufficiency in Italy: the 24-cities cohort study. Eur J Vasc Endovasc Surg. 2005;30:422-429.
12. Zahariev T, Anastassov V, Girov K, et al. Prevalence of primary chronic venous disease: the Bulgarian experience. Int Angiol. 2009;28:303-310.
13. McLafferty RB, Passman MA, Caprini JA, et al. Increasing awareness about venous disease: The American Venous Forum expands the National Venous Screening Program. J Vasc Surg. 2008;48(2):394-399.
14. Marone EM, Volontè M, Limoni C, et al. Therapeutic options and patterns of prescription in chronic venous disorders: Results of a 3-year survey in Italy. Eur J Vasc Endovasc Surg. 2009;38;511-517.
15. Agus GB, Mondani P, Ferrari P, et al. New epidemiology. The impact of CEAP classification and style of life on chronic venous insufficiency. Acta Phlebol. 2000;1:7-15.
16. Agus GB, Allegra C, Arpaia G, et al. Linee guida diagnostico-terapeutiche delle malattie delle vene e dei linfatici. Acta Phlebol. 2000;1(suppl. 1):1-55. Revised version: Acta Phlebol. 2003;4:1-52. Revised English version: Int Angiol. 2005;24:107- 168.
17. Allegra C. Chronic venous insufficiency: the effects of health-care reforms on the cost of treatment and hospitalization. An Italian perspective. Curr Med Res Opinions. 2003;19:761-769.
18. Garde C. Les phlébotoniques en question. Phlébologie. 2003;56:101-2.
19. WHO. Preventing chronic diseases: a vital investment. WHO global report. Geneva. World Health Organization. 2005.
20. Korn P, Patel ST, Heller JA, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg. 2002;35:950-957.
21. Ernst E, Saradeth T, Resch KL. Hydrotherapy for varicose veins: a randomized, controlled trial. Paris, France: John Libbey Eurotext; 2003:944- 946.
22. Garde C. Le drainage veineux manuel. Phlébologie. 1992:948-951.
23. Abu-Own A, Scurr JH, Coleridge Smith PD. Effect of leg elevation on the skin microcirculation in chronic venous insufficiency. J Vasc Surg. 1994;20:705.
24. Ohgi S, Tanaka K, Maeda T, et al. Comparison of three exercises for evaluation of the calf muscle pump. Phlebology. 1995;10:23-27.
25. Padberg FT, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: A randomized trial. J Vasc Surg. 2004;39:79-87.
26. Peresa M, Krajcar J. Troubles de la statique du pied et insuffisance veineuse chronique. Phlébologie. 1987;40:1029- 1037.
27. Brizzio OE, Bacci PA, Pierguidi P. Il ruolo del plantare flebo logico. Int Angiol 1997;16(suppl. 1):38.
28. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44:136- 144.
29. Bergan JJ, Schmid-Schönbein GN, Coleridge Smith P, et al. Chronic venous disease. N Engl J Med. 2006;355:488-498.
30. Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96:1231-1242.
31. Lim CS, Shaloub J, Gohel MS, et al. Matrix metalloproteinases in vascular disease. A potential therapeutic target? Curr Vasc Pharmacol. 2010;8:75-85.
32. Simionescu M, Simionescu N. Functions of the endothelial cell surface. Ann Rev Physiol. 1986;48:279-293.
33. Gohel MS, Davies AH. Pharmacological treatment in patients with C4, C5 and C6 venous disease. Phlebology. 2010;25(suppl 1):35-41.
34. Cyrino FZ, Lerond L, Bouskela E. Micronization enhanced the protective effect of Purified Flavonoid Fraction against post-ischemic microvascular injury in hamster cheek pouch. Clin Experim Pharmacol Physiol. 2004;31:159- 162.
35. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41:117-125.
36. Domínguez C, Brautigam I, González E, et al Therapic effects of hidrosmin on chronic venous insufficiency of the lower limbs. Curr Med Res Opin. 1992;12:623- 630.
37. Gilly R, Pillion G, Frileux C. Evaluation of a new veno active micronized Flavonoid- Fraction (S 5682) in symptomatic disturbances of the venolymphatic circulation of the lower limb: a doubleblind, placebocontrolled study. Phlebology. 1994;9:67-70.
38. Ramelet AA. Clinical benefits of MPFF at a dose of 500 mg in the most severe stages of chronic venous insufficiency. Angiology. 2001;52(suppl 1):S49-S56.
39. Poynard T, Valterio C. Meta-analysis of hydroxyethylrutosides in the treatment of chronic venous insufficiency. Vasa. 1994;23:244-250.
40. Vin F, Chabanel A, Taccoen, et al. Double blind trial of the efficacity of troxerutin in chronic venous insufficiency. Phlebology. 1994;9:71-76.
41. Petruzzellis V, Troccoli T, Candiani C, et al. Oxerutins: efficacy in chronic venous insifficiency. A double-blind, randomized, controlled study. Angiology. 2002;53:257- 263.
42. Nicolaides AN. Chronic venous disease and the leukocyte- endothelium interaction: from symptoms to ulceration. Angiology. 2005;56(suppl 1):11-19.
43. Bergan JJ, Pascarella L, Schmid- Schönbein G. Pathogenesis of primary chronic venous disease: insights from animal models of venous hypertension. J Vasc Surg. 2008;47:183-192.
44. Blume J, Langenbahn H, de Champvallins M. Quantification of edema using the volometer technique: therapeutic aplication of MPFF at a dose of 500 mg in chronic venous insufficiency. Phlebology. 1992;suppl 2:37-40.
45. Kearon C, Kahn SR, Agnelli GC, et al. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence- Based Clinical Practice Guidelines. 8th ed. Chest. 2008;133;454-545.
46. Jantet G. Chronic venous insufficiency: worldwide results of the RELIEF Study. Angiology. 2002;53:245-256.
47. Launois, R, Mansilha, A, Jantet, G. International psychometric validation of the chronic venous disease quality of life questionnaire CIVIQ-20. Eur J Vasc Endovasc Surg. 2010;40:783-789.
48. Ramelet AA, Boisseau MR, Allegra C, et al. Veno-active drugs in the management of chronic venous disease. An international consensus statement: Current medical position, prospective views and final resolution. Clin Hemorheol Microcirc. 2005;33:309-319.
49. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Review). Cochrane Library. 2007;Issue 2.
50. Martinez MJ, Bonfill X, Moreno RM, et al. Phlebotonics for venous insufficiency (Review). Cochrane Library. 2007;Issue 2.
51. Agus GB, Allegra C, Arpaia G et al. Guidelines on compression therapy. Acta Phleb. 2001;2(suppl 1):3-24.
52. Partsch H. Evidence based compression therapy. Vasa. 2003;32(S63):3-39.
53. Cornu-Thénard A, Benigni JP, Uhl JF, et al. Recommandations de la Société Française de Phlébologie sur l’utilisation quotidienne de la thérapeutique compressive. Phlébologie. 2006;59:237- 244.
54. Mariani F. Consensus conference on compression therapy. Torino: Minerva Medica. 2009.
55. Andreozzi GM, Cordova R, Scomparin MA, et al. Quality of life in chronic venous insufficiency. An Italian pilot study of the Triveneto Region. Int Angiol. 2005;24:272-277.
