Chronic venous disease and the genetic influence
Department of Pharmacology, Bordeaux,
France
ABSTRACT
The etiology and pathophysiology of chronic venous disease (CVD) have been intensively studied in the past decades. The elucidation of mechanisms leading to CVD is a challenging task because acquired phenomena alone are presumably insufficient to explain the full clinical course of the disease. CVD genetic screening adopted the most appropriate methodology to find out which chromosomal and/or gene defects could be responsible for the predisposition. They include family studies, gene expression methods (eg, single candidate gene expression, DNA microarrays), or genotyping methods (eg, single nucleotide polymorphisms, mutations). Investigations have so far only partially produced coherent results and there must be further collaborations in order to successfully advance the field of venous disease genetics. More acute definitions and better classification of patients should avoid major bias. The present review offers a panoramic view on the genetic propensity of venous disorders based on literature.
The venous system in humans is permanently submitted to high blood pressures, particularly in lower limb veins. Prolonged venous hypertension, due to standing, unknown among other mammalians, is responsible for diverse clinical aspects of chronic venous disease (CVD), mostly of primary etiology. Globally, CVD may affect more than 60% of the adult population,1and its high costs, both at individual and societal levels, have been well documented.2
Research in recent years has dramatically improved our understanding of the pathophysiology of CVD. Prolonged venous hypertension is linked to all theories regarding CVD pathogenesis and chronic inflammation, which maintains a vicious circle of disease progression. Numerous inflammatory factors, cytokines, growth factors, and tissue enzymes such as vascular endothelial growth factor (VEGF), tumor growth factor β(TGF β), and tissue inhibitors of the matrix metalloproteinases (TIMPs) are involved in the loop, which ends with remodeling and fibrosis of the venous wall. These complex interacting events have been extensively studied.3-5 Chronic hypertension is transmitted to the capillaries, which ultimately leads to ulceration6 (see below for more details).
Whether CVD is mostly acquired rather than partly due to hereditary phenomenon remains to be elucidated. The aim of this review is to discuss the available data from literature concerning heredity of varicose veins from epidemiology to molecular disturbances and venous ulcers including their quality of healing.
HEREDITY OF VARICOSE VEINS
Social and environmental factors
CVD evolution, at any stage, is linked to intricate social or environmental factors and to the field of physiology and biology. For example, obesity, which increases pressure in lower limbs veins, is linked to genetic factors because the heritability is close to 80%.7,8 However, obesity is also linked to social food habits or may even be due to adenovirus infections or trendy eating fashions. In addition, fat tissue in obese patients usually delivers plasminogen activator inhibitor (PAI)-1, interleukin-6, and the adhesion molecule P-selectin, which all contribute to vein lesions. From a genetic point of view, these factors can all be altered by gene defects. CVD exhibits “multisided clinical phenotypes; characteristics of complex diseases.”9
Large epidemiological surveys
Some studies have reliably examined the role of genetic factors in the overall clinical picture, but no study has examined it at the molecular level. For a long time, physicians have asserted that 70% to 80% of their patients with varicose veins have a family history of the disease,10-12 and it was found that 42% of women with varicose veins had a family history.13 In a study that analyzed 4033 nuclear families, heritability of CVD equaled 17.3%, suggesting a genetic component.14
Twins
Few studies have been conducted on twins. Available data shows a higher degree of concordance between monozygotic twins (75%) than between dizygotic twins (52%), although the difference was not statistically significant because the numbers involved were too few.15 In a study based on impedance plethysmography in 46 pairs of twins, it was shown, after appropriate adjustments, that heritability of compliance was 90%; so a venous physical function, involved in the wall tonicity, is dependent on genetic factors.16
Case-control studies
A case-control study conducted on 134 families has properly analyzed the incidence of varicose veins in one or more of the parents examined clinically; this latter point being essential and far better than using questionnaires. The risk of developing varicose veins for the children was 90% when both parents suffered from this disease, and 25% for males and 62% for females when one parent was affected.17 Using the Hospital Register in Sweden, and thereby eliminating recall bias, family history of hospital treatment for varicose veins was associated with an increased risk of similar treatment among relatives.18 In an assay conducted in Finland, among a large population of people between 40 and 60 years of age, a reported positive family history of varicose veins was found to be an independent and significant risk indicator.19 In another trial, 82% of patients with CVD and 22% without had a positive family history.20 Misclassification of varicose veins may have introduced some bias in such studies, depending on whether ‘varicose veins’ were defined as visible dilated veins or as refluxing veins.21,22
Modes of heredity
Various modes of heredity have been hypothesized. Some authors have, from the outset, postulated a recessive mode of inheritance, which involves a pedigree segregation ratio as well as a discontinuous mode of heredity. Moreover, when only one parent was affected, no correlation was observed between the sex of the affected parent and varicose veins in the children. However, it was common to observe pedigrees where men and their fathers, but not their mothers, were affected. These factors tend, therefore, to exclude a sex-linked pattern of inheritance and to stress the multifactorial nature of varicose vein inheritance.23 However, the data from the literature seem to indicate the existence of different types of heredity depending on the families studied.
Other investigators observed an autosomal dominant like inheritance with an estimated 50% of varicose vein patients having some genetic linkage.24 A Chinese study investigated the hereditary nature of varicose veins in a hospital population.25 The analysis of nuclear families was compatible with autosomal dominant inheritance, with 70% to 92% penetrance, some pedigrees were compatible with autosomal recessive inheritance, and 37% of cases were sporadic.
Recently, an autosomal dominant mode of inheritance has been observed in nine families.26 Varicose vein disease in these families was linked to the candidate marker D16S520 on chromosome 16q24, which may account for the linkage to the transcription factor fork head box protein C2 (FOXC2). Mutations in the FOXC2 gene are associated with the lymphedema-distichiasis syndrome, and varicose veins are commonly observed as one of its phenotypic abnormalities at an early age.27 Such findings suggest a possible role for the FOXC2 gene in the pathogenesis of varicose vein formation and lymphatic dysfunction in such families.
Ethnicity
In some studies, the ethnic origin of subjects has been taken into account. In the San Diego multiethnic cross– sectional study on 2211 persons, CVD appeared to be more common in non-Hispanic whites than in Hispanics, African-Americans, and East Asians.28 Though such results were obvious, they appear likely to be linked with social habits rather than genetics. In a prospective cohort study performed in aged residents, African- Americans exhibited higher levels of IL6, which it is considered to be a marker of both CVD and ulcers,29 but in fact, they represented a group of obese patients, heavy smokers, etc. So a socially disadvantaged environment seems to be a main cause of CVD occurrence when ethnicity is taken into account, with the more possible underlying genetic factors being masked and not easily diagnosed. It should be noted that in the past, African tribes or other ethnic races were considered to have less prevalence of varicose veins that Europeans; however, that was related to the absence of sitting on chairs. After the use of tables and chairs were introduced to African families, the difference between African tribes and Europeans has disappeared.
Family history of varicose veins with mast cell infiltration
In one study, members of families with CVD exhibited higher mast cell counts in the adventitia of the varicose vein as a regular histological observation.30
Blood groups and varicose veins–a weak link
A case-control study, including 395 subjects, found a relationship between the A blood group and the presence of varicose veins, as well as a relation with vein thrombosis occurrence in non-O blood groups. It remains difficult, however, to keep the A blood group as an efficient risk factor of CVD!31
In spite of the numerous nosological restrictions of studies focusing on the hereditary aspect of varicose veins, it can nevertheless be safely said that there is a significant genetic factor. However, its significance is interpreted in different ways and the nature of the genetic factor is still a topic of debate: is it due to heredity, family habits, or genetic transmission, and if so, by which genetic mode?
Some general metabolic inherited disorders are known to constitute a background for alteration of tissues and organs, mainly with a link to vascular diseases and thrombosis.
Hyperhomocysteinemia
An increased level of plasma homocysteine is found in 65% of patients with CVD. Increased homocysteine levels are correlated with the CEAP classification grades, favors venous wall changes, and are also a risk factor for venous thrombosis. Prevalence of the 677C>T mutation in methylene tetrahydrofolate reductase was higher at the C4-C6 stages (20%) than in earlier stages (10%). It appears that around 15% of patients were homozygous, compared with 5% in a “healthy” white population.32
Unbalanced collagen types in the venous wall
Sansilvestri-Morel et al demonstrated that dermal fibroblasts from both the vein wall and the skin of subjects with varicose veins have an altered collagen profile.33 The clinically observed vessel wall thickening appears to be associated with an increase in thick and disorganized collagen bundles.34 Varicose veins are characterized by a smooth muscle cell and extracellular matrix component disorganization in the venous wall, which is associated with abnormal distensibility of varicose veins. The level of collagen type III is decreased in cultured smooth muscle cells and dermal fibroblasts derived from patients with varicose veins and hydroxyproline is overproduced in smooth muscle cells suggesting increased collagen content. This collagen augmentation appears to be correlated with an increase of collagen type I. Since collagen type I confers rigidity and collagen type III provides distensibility in tissues, such changes could contribute to the weakness and reduced elasticity of varicose veins. In conclusion, the collagen III defect seems to be generalized in different tissues and argues in favor of a genetic alteration in wall remodeling when submitted to prolonged pressure.35,36 The importance of such a systemic defect in the connective tissue has been stated in consensual reviews.5,6 Unfortunately, there is a lack of epidemiological data related to the lesion frequency in a large population, which could be due to ethical problems and the need for skin biopsies.
Alleged genetic influence on wall remodeling
An imbalance in matrix metalloproteinases (MMPs) and TIMPs was observed in patients with varicose veins, which could be modulated genetically.34 Altered apoptosis, either enhanced37 or decreased,38,39 in the varicose vein wall has been described and such results could be influenced by frequent single nucleotide polymorphisms (SNPs) on genes involved in apoptosis pathways. An observed down regulation of desmuslin, an intermediate filament protein, in smooth muscle cells of varicose veins may be due to defects in related structural genes.40 Additionally, the thrombomodulin 1208/12O9TT deletion mutation has been associated with vein thrombosis and subsequently with varicose veins.41
Hemochromatosis factor XIII variants in the evolution of CVD
The HFE C282Y hemochromatosis gene mutation and factor XIII V34L gene variants have been identified in patients with varicose veins and may have long term implications for increased risk of more severe forms of CVI.42,43 The main role of these factors appear to be in the ulcer formation as described below in detail.
The occurrence of varicose veins can be related to specific defects or well defined gene mutations, allowing groups of patients with CVD to be specifically included in defined syndromes.
CADASIL (Notch 3 gene mutation)
A heterogeneous mutation on the Notch 3 gene has been identified in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). In the CADASIL pedigree with varicose veins, a mutation of the Notch 3 gene (at the 3’ acceptor site of intron 15) causes degeneration of venous smooth muscle cells that in turn can generate CVD.44
FOXC2 mutations with varicose veins
FOXC2 is an important gene for the development of venous and lymphatic valves. Mutations in the FOXC2 gene cause lymphedema distichiasis, which is often associated with varicose veins. These mutations are strongly related to venous valve failure.45 Patients who have a homozygous cytosine to thymine mutation at position 598 (598C>T) of the von Hippel-Lindau gene (VHL) are likely to have varicose veins. This mutation is also linked to increased expression of hypoxia inducible factor-1 (HIF-1) genes, which induce VEGF and transferrin receptors (TfR).46
Angiodysplasias
Ehlers-Danlos syndrome comprises more than 10 types of connective tissue disorders with abnormal collagen synthesis. The syndrome is characterized by joint hypermobility, distensible skin, ocular disease, bone deformities and fragility, as well as cardiovascular disorders that render blood vessels and visceral tissue walls fragile and susceptible to rupture. Patients with Ehlers-Danlos syndrome type IV, which predisposes them to vascular pathologies, may present with varicose veins,6,47 while surprisingly, the Marfan disease, another connective tissue syndrome, only affects the arterial system.6 In the congenital Klippel-Trenaunay syndrome, the patient presents with varicose veins, limb hypertrophy, and dermal capillary hemangioma (port wine stain),6 and 75% to 100% of patients exhibit venous valve hypoplasia.
A mutation of the Norrie disease gene (ND), an X-linked recessive disorder, is responsible for ocular anomalies and further sensory neural deafness, which develops by the second decade in up to 100% of individuals. The ND gene mutation can be associated with peripheral vascular diseases such as varicose veins, venous ulcers, and erectile dysfunction. It is present in nearly all males over the age of 50, perhaps as a result of small vessel angiopathy, and its age of onset is similar to that of the hearing deficit and the time course of progression is similar.48
Finally, 82 upregulated genes belonging to extracellular matrix molecules, cytoskeletal proteins, and myofibroblasts were identified in varicose veins using a cDNA microarray. These genes include: transforming growth factor 3-induced gene (BIGH3), tubulin, lumican, collagen type I, versican, actin, and tropomyosin.49
Table I summarizes the hereditary factors in varicose veins.
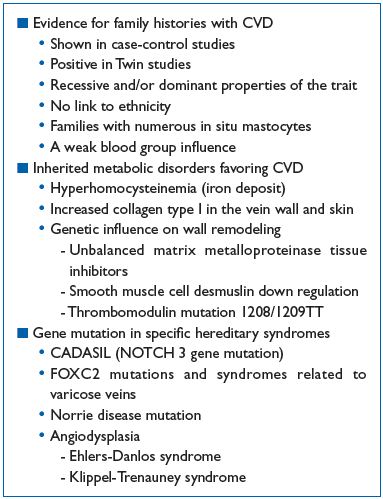
Table I. Hereditary factors and varicose veins
HEREDITARY FACTORS IN THE OCCURRENCE OF VENOUS ULCERS
Epidemiological studies underline the prevalence of venous ulcers in around 0.3% of CVD patients, and that healed ulcers are observed in 1% of the adult population both in Europe and the USA.50 Few data are available regarding family history, ethnic groups, and rapid ulcer occurrence. In a large review of longitudinal studies, the role of obesity in the occurrence of ulcers is identified, especially in the Framingham Study and the Bonn Study.51
In the West London Study, regarding patients presenting with leg ulcer(s) at their consultations over a oneyear period, the prevalence of ulcer(s) were higher in white people than in a South Asian population (odds ratio=4.43), but the authors themselves pointed out a bias in recruitment.52
Previous history of DVT, age, arterial hypertension, and lifestyle (prolonged standing) are main actors in the development of ulcers, which constitutes a “cloud” preventing the discovery of potential underlying putative genetic factors. Particularly, it is unknown how many patients with superficial reflux will progress to venous ulceration.50 Is venous ulceration, due to lifestyle, lack of exercise, family habits, or is there a genetic factor involved? The debate is still ongoing.
There is, however, an exception. Patients with homozygous sickle cell disease (homozygous for the hemoglobin mutation 6 glu>val; HbSS), frequently suffer from venous ulcerations of the lower limbs, which are often disabling. The mechanism appears complex and not related to venous disease by itself, but to arteriovenous shunting along with an abundance of hypoxic cells.53
Mechanism of the lower limb venous ulcer development
The ulcer develops over skin regions with predictive aspects such as corona phlebectatica, a varicose edema occurring mainly at advanced stages of the CEAP classification. Using capillaroscopy at the ankle, microvascular ischemia combined with venous and lymphatic edema, define areas of the skin where ulcers develop.54 Inside the skin, the setting of previous biological events can also predict sites of ulcer development and are marked by: increased permeability, erythrocyte migration, iron deposits leading to free radical activity in the tissue, leukocyte migration (white cell trapping), and migration of macrophages and mastocytes.
The mechanism of ulcer formation is shown in Figure I. Two processes act separately:
1. Necrosis of the extracellular matrix, in which TGFβ, proteolytic hyperactivity of MMPs, and destruction of collagen microfibrils and proteoglycans (opening craters in the skin) play a role.4,5
2. Increased apoptosis of keratinocytes and caspase-2 activation leads to dermo-epidermal detachment that eventually progresses to an ulcer.55
Many biological factors and/or processes lead to ulcer formation, and are influenced, sometimes strongly), by the patient’s heredity (ie, either an abnormal or dysfunctional gene due to a close acting SNP. The situations are diverse and implies either large metabolisms or precise genetic processes.
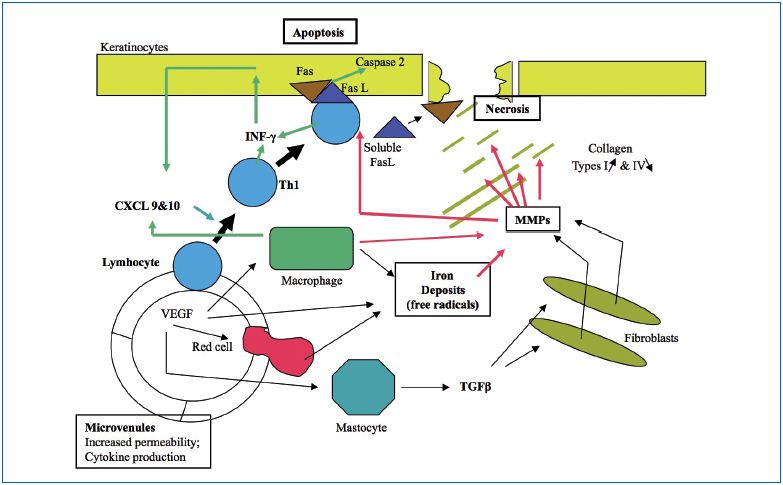
Figure 1. Venous ulcer , 5, 55
Iron deposition is correlated with the occurrence of skin complications in chronic venous insufficiency (CVI). It was recently demonstrated that visible iron deposits cause lesions in some, but not all, individuals due to functional iron and related gene variants. As previously stated, a dysregulated iron cycle leads to local iron overload that could generate free radicals or activate a proteolytic hyperactivity on the part of MMPs or downregulate tissue inhibitors of MMPs.
Iron cycle
Figure 2 underlines the role of the membrane-linked HFE protein opposing TfR-1 that controls iron influx through ferroportin, however, other actors are potent players. Hepcidin, a low molecular weight protein, shuts down the iron influx by engulfing ferroportin at the intestinal barrier and from macrophages, which are also an important source of ferritin deposit in tissues. In the end, iron influx is blocked first by the receptor HFE and second by Hepcidin. Hepcidin regulators in the liver may be dysfunctional.
Familial Hemochromatosis
Hereditary hemochromatosis is incorrectly assumed to be due to a single gene. Actually, the overwhelming majority actually depends on mutations in the HFE gene, but there is non-HFE–related hemochromatosis. One can summarize as follows, excluding some other close SNPs influences.
1. Hemochromatosis Type 1:
• Frequent
• Autosomal recessive inheritance
• Gene HFE mutations: HFE C282Y and HFE H63D
2. Hemochromatosis Type 2A:
• Juvenile, rare
• Autosomal recessive inheritance
• Hemojuvelin decreases due to a HFE-2 gene mutation
3. Hemochromatosis Type 2B:
• Juvenile, rare
• Autosomal recessive inheritance
• Hepcidin decreases due to a Hamp gene mutation.
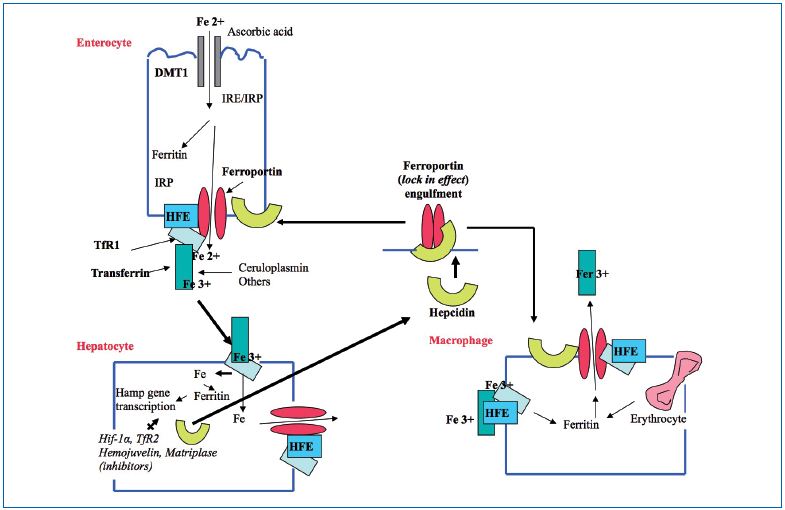
Figure 2. Iron transport: opposing effects of the proteins HFE and Hepcidin
4. Hemochromatosis Type 3:
• Juvenile, rare
• Autosomal recessive inheritance
• TfR-2 gene mutation leading to dysfunctional transferrin and Hepcidin
5. Hemochromatosis Type 4:
• Juvenile, African, rare
• Autosomal dominant inheritance
• Lack of ferroportin, due to a FNP1 gene mutation (SLC40A1)
HFE C282Y is very frequent in Europe and increases, by a factor of 7, the risk of developing a venous ulcer. Despite the low penetrance of the gene, its influence is high even in heterozygotes. The membrane bound proteins related to the HFE C282Y gene are no longer able to lock out the ferroportin mechanism (Figure 2).
In a case-control study, among 980 patients with severe CVD (C4-C6), 518 cases were genotyped for HFE mutations. The C282Y mutation significantly increases, by a factor of 7, the risk of an ulcer in primary CVD (odds ratio=6.69) by 7. Application of the research demonstrated an increased specificity (98%) and a positive value (86%).42 The HFE H63D variant is rare, but ulcers form occur 10 years earlier in patients with CVD.9,56 On the whole, including double heterozygotes, 10% of patients might be affected by hemochromatosis gene mutations.42,57
SNP on the ferroportin gene FPN1
Few data are available regarding studies related to SNPs and ulcer occurrence. Recently, a significant association was identified between FPN1-8CG, a SNP in the promoter region of the FPN1 gene, and ulcer susceptibility, showing that susceptibility increased 5-fold.9 The FPN1-8CG polymorphism is close to the region of iron response element (IRE), and could act at macrophage membranes, paradoxically hampering efflux of iron from the cell, but leading to macrophage death. In this study, the risk of an ulcer appeared to be in addition to the HFE C282Y gene (3% of individuals).
An elegant study showed that SNPs in the Estrogen receptor beta (ERβ) gene was associated with venous ulceration in elderly patients (SNPs close to the regulatory region of the ERβ gene including the ON exon and promoter). Such SNPs were found in 23% of the elderly persons investigated. A major susceptibility in haplotype, carried by 23% of cases, was significantly associated with an active ulcer. Furthermore, there was an association with elevated serum levels of tumor necrosis factor α. In such groups of patients, the “dampening” effect of estrogen on inflammation is decreased, thereby facilitating an ulcer, both in genesis and healing.58
In a study performed on 88 patients with a venous ulcer, 41% have a thrombophilia prevalence rate that is 30 times higher than the rate of the general population.59 Hemostasis disorders were quite diverse: antithrombin and protein S, C deficiencies, activated protein C resistance, factor V Leyden, presence of prothrombin 20210A, lupus anticoagulant, and cardio lipid antibodies. There was no relation with previous deep venous thrombosis. The prevalence of venous ulcers in patients having factor V Leyden had been previously reported.60 It remains difficult to assert that coagulation disorders could play an actual role in ulcer formation, knowing that most of them, like factor V Leyden, are weak risk factors in thrombosis. A role in poor healing could be a more convincing possibility.
The proteolytic enzymes, MMP-1, -2, -8, and -9, play an important role in ulcer formation due to their ability to degrade extracellular matrix components (Figure 1). Particularly efficient is an imbalance in MMPs versus TIMPs-1, -2, and -3 activity ratios, and HIF-1β enhances their effects. MMP SNPs have been studied in several diseases, but there were only a few with regards to ulcer formation. Polymorphisms in the promoter regions of MMP-2, -9, and -12 have been studied in diseases where ECM destruction is an important process, (eg, diabetes, coronary arterial disease),1,62 and recently it has been shown that the polymorphism MMP12-82AG, located in the coding region of the gene, induces a 2-fold higher risk of developing a venous ulcer in patients with primary CVD.9
Tumor Necrosis Factor α polymorphism, the 308G>A polymorphism in the promoter region, has been associated with ulcer susceptibility.63 It has been suggested to have a possible association with obesity.64 Other cytokine SNPs (eg, VEGF isoforms) are likely to be involved in the evolution, onset, and duration of venous ulcers, and some particular roles in apoptosis.
Table II summarizes the hereditary factors in venous ulcer formation.
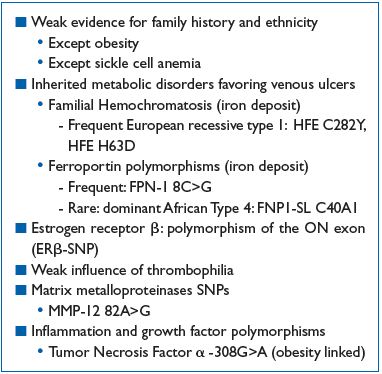
Table II. Hereditary factors and venous ulcers
VENOUS ULCER HEALING AND GENETICS
Figure 3 shows the main factors leading to ulcer healing. The ulcer crater is recovered by a layer of thrombin induced fibrin which must be of normal quality. The ECM must be rebuilt by fibroblasts with a normal balance between collagen I and III. Growth factors (eg, platelet factor 4, VEGF, etc) are needed, keratinocytes must proliferate, and the superficial lining of cells must be rebuilt. This latter process requires apoptosis to be stopped. A certain number of abnormalities with a link to the personal heredity in given patients have been reported.
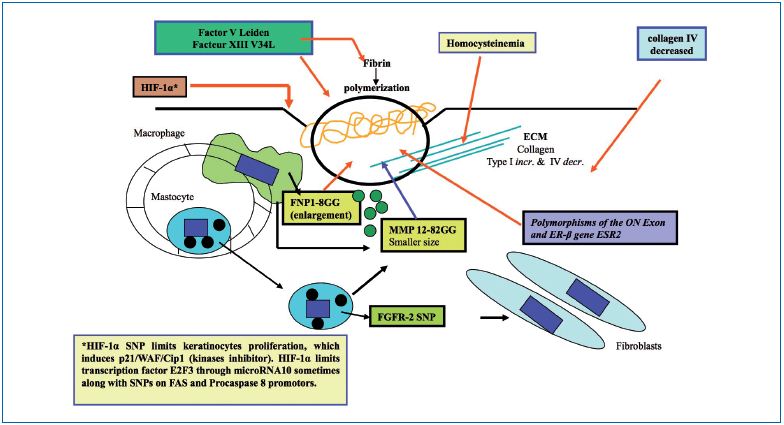
Figure 3. Venous ulcer healing and genetics
Thrombophilia could impair ulcer healing, and at the same time, favor its incidence. Such an assertion is poorly documented because fibrin in the ulcerous crater is due to tissue factors coming from altered neighboring tissues, without the involvement of the intrinsic coagulation cascade. There is a need for an efficient fibrin polymerization by factor XIII to improve fibroblast migration, and so the dysfunctional factor XIII-SNP-V34L has been shown to impair healing.43,65
Collagen IV deficiency and high levels of homocysteine are implicated in reduced ulcer healing.
In elderly patients, the ERβ-SNP (ON exon) is also a cause of poor venous ulcer healing.58
The fibroblast growth factor receptor 2-SNP is often present in CVD patients with venous ulcer and is also a cause for poor healing.64
Approximately, 50% of humans would have SNPs in the extrinsic apoptotic chain, according to many researchers in cancer, diabetes, and immunology fields. Although these SNPs have not specifically been found in the ulcer, we can speculate about a possible role for either increasing or decreasing the intensity of keratinocyte destruction. On the FAS ligand (FAS-L) promoter, the FAS-SNP-844T>C has been identified, which favors its induction (42% homozygotes and 9% heterozygotes). FAS promoter SNPs are able to diminish FAS-L joining and the same is true for the procaspase-8 promoter. Other proteins are involved in apoptosis such as the Bcl- 2 family where other important SNPs could be located. HIF-1α, an important transcription factor, is induced by both hypoxia and oxidative stress. HIF-1α may contain two important SNPs (1772C>T and 179OG>A) that leads to an increased transactivation capability and may play a role in colorectal cancer.66 Hypoxemia in the crater of an ulcer can induce HIF-1α, and subsequently, keratinocyte regeneration would dramatically decrease by up regulating the kinase inhibitor p21.67
Some inverse observations showed that specific FXIII genotypes, such as FXIII V34L, also evaluated in venous ulcer healing following superficial venous surgery in patients with CVD, promote favorable ulcer healing rates. However, the HFE gene mutation, despite its importance in venous ulcer risk, had no influence on healing time. Also, it has been reported that homozygous MMP-12 SNP-82GG favors a smaller ulcer size.9
Table III summarizes the influence of genetics in venous leg ulcer healing.
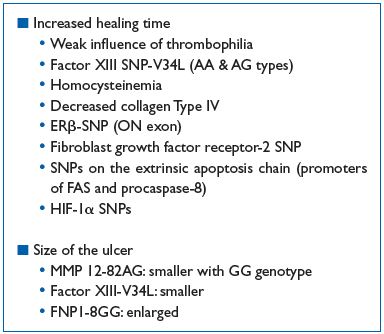
Table III. Hereditary factors and venous ulcer healing
CONCLUSION
The list of hereditary factors, as discussed in this review, that influence the quality of the venous wall in CVD and the formation of an ulcer, is far from being complete. SNP discoveries will continue, but above all, there is a particular need for cross-sectional epidemiology studies in order to unveil the actual role of heredity, which is masked by a “cloud” of lifestyle and acquired factors. Critical functions of susceptibility genes and the translational pathways leading to the observed CVD or venous ulcer should be analyzed. Metabolomic technologies could help identify the metabolic pathways involved in varicose veins and how they are controlled by both genetic and environmental factors.68 MicroRNA profiles of diseased veins could also help understand the remodeling pathways.69

REFERENCES
1. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31:105-115.
2. Nicolaides AN, Allegra C, Bergan J, et al; American venous forum. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1-59.
3. Schmid-Schonbein GW. Molecular basis of venous insufficiency. In: Bergan J, ed. The Vein Book. London, UK: Elsevier Academic Press; 2007:67- 78.
4. Pappas PJ, You R, Rameshwar P, et al. Dermal tissue fibrosis in patients with chronic venous insufficiency is associated with increased transforming growth factor-beta1 gene expression and protein production. J Vasc Surg. 1999;30:1129-1145.
5. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355:488-498.
6. Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23:85-98.
7. Bell CG, Walley AJ, Froguel P. The genetics of human obesity. Nat Rev Genet. 2005;6:221-234.
8. Kostas TI, Ioannou CV, Drygiannakis I, et al. Chronic venous disease progression and modification of predisposing factors. J Vasc Surg. 2010;51:900-907.
9. Gemmati D, Federici F, Catozzi L, et al. DNA-array of gene variants in venous leg ulcers: detection of prognostic indicators. J Vasc Surg. 2009;50:1444- 1451.
10. Leu HJ. Heredity of varicose disease. Rev Medicine. 1966;9:467-471.
11. Merlen JF, Coget J, Larere J. Heredity of varices. Phlebologie. 1967;20:213- 216.
12. Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15:175-184.
13. Hirai M, Naiki K, Nakayama R. Prevalence and risk factors of varicose veins in Japanese women. Angiology. 1990;41:228-232.
14. Fiebig A, Krusche P, Wolf A, et al. Heritability of chronic venous disease. Hum Genet. 2010;127:669-674.
15. Niermann H: Zwillingsdermatologie. 1 Vol Berlin, Springer-Verlag, 1964, p 32-33.
16. Brinsuk M, Tank J, Luft FC, Busjahn A, Jordan J. Heritability of venous function in humans. Arterioscler Thromb Vasc Biol. 2004;24:207-211.
17. Cornu-Thenard A, Boivin P, Baud JM, De Vincenzi I, Carpentier PH. Importance of the familial factor in varicose disease. Clinical study of 134 families. J Dermatol Surg Oncol. 1994;20:318-326.
18. Zoller B, Ji J, Sundquist J, Sundquist K. Family history and risk of hospital treatment for varicose veins in Sweden. Br J Surg. 2012;99:948-953.
19. Laurikka JO, Sisto T, Tarkka MR, Auvinen O, Hakama M. Risk indicators for varicose veins in forty- to sixtyyears- olds in the Tempere varicose vein study. World J Surg. 2002;26:648-651.
20. Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risks factors for chronic venous insufficiency: a dual casecontrol study. J Vasc Surg. 1995;22:622- 628.
21. Pistorius MA. Chronic venous insufficiency: the genetic influence. Angiology. 2003;54:S5-S12.
22. Ahti TM, Makivaara LA, Luukkaala T, Hakama M, Laurikka JO. Effect of family history on the risk of varicose veins is affected by differential misclassification. J Clin Epidemiol. 2010;63:686-690.
23. Gundersen J, Hauge M. Hereditary factors in venous insufficiency. Angiology. 1969;20:346-355.
24. Matousek V, Prerovsky I. A contribution to the problem of the inheritance of primary varicose veins. Hum Hered. 1974;24:225-235.
25. Guo Q, Guo C. Genetic analysis of varicose vein of lower extremities. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 1998;15:221-223.
26. Serra R, Buffone G, de Franciscis A, et al. A genetic study of chronic venous insufficiency. Ann Vasc Surg. 2012;26:636-642.
27. Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedemadistichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J Med Genet. 2002;39:478-483.
28. Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003;158:448-456.
29. Purser JL, Kuchibhatla MN, Miranda ML, Blazer DG, Cohen HJ, Fillenbaum GG. Geographical segregation and IL6: a marker of chronic inflammation in older adults. Biomark Med. 2008;2:335- 348.
30. Kakkos SK, Zolota VG, Peristeropoulou P, Apostolopoulou A, Geroukalos G, Tsolakis IA. Increased mast cell infiltration in familial varicose veins: pathogenetic implications? Int Angiol. 2003;22:43-49.
31. Degrave-Contour N. Le groupe sanguin A comme facteur de risque des varices des membres inferieurs : une enquete cas-temoins 395 cas. These pour le Doctorat en Medecine. Faculte de Medecine Saint Antoine, Paris 1987. BDSP, ref 19529.
32. Sam RC, Burns PJ, Hobbs SD, et al. The prevalence of hyperhomocysteinemia, methylene reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. J Vasc Surg. 2003;38:904- 908.
33. Sansilvestri-Morel P, Rupin A, Jaisson S, Fabiani JN, Verbeuren TJ, Vanhoutte PM. Synthesis of collagen in dysregulated cultured fibroblasts derived from skin of subjects with varicose veins as it is in venous smooth muscle cells. Circulation. 2002;106:479- 483.
34. Sansilvestri-Morel P, Fioretti F, Rupin A, et al. Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Science. 2007;112:229- 239.
35. Sansilvestri-Morel P, Rupin A, Badier- Commander C, Fabiani JN, Verbeuren TJ. Chronic venous insufficiency: dysregulation of collagen synthesis. Angiology. 2003;54:S3–8.
36. Sansilvestri-Morel P, Rupin A, Jullien ND, et al. Decreased production of collagen type III in cultured smooth muscle cells from varicose vein patients is due to degradation by MMPs: possible implication of MMP-3. J Vasc Res. 2005;42:388-398.
37. Filis K, Kavantzas N, Isopoulos T, et al. Increased vein wall apoptosis in varicose vein disease is related to venous hypertension. Eur J Vasc Endovasc Surg. 2011;41:533-539.
38. Ascher E, Jacob T, Hingorani A, Tsemekhin B, Gunduz Y. Expression of molecular mediators of apoptosis and their role in the pathogenesis of lowerextremity varicose veins. J Vasc Surg. 2001;33:1080-1086.
39. Ducasse E, Giannakakis K, Speziale F, et al. Association of primary varicose veins with deregulated vein wall apoptosis. Eur J Vasc Endovasc Surg. 2008;35:224-229.
40. Yin H, Zhang X, Wang J, et al. Downregulation of desmuslin in primary vein incompetence. J Vasc Surg. 2006;43:372-378.
41. Le Flem L, Mennen L, Aubry ML, et al. Thrombomodulin promoter mutations, venous thrombosis, and varicose veins. Arterioscler Thromb Vasc Biol. 2001;21:445-451.
42. Zamboni P, Tognazzo S, Izzo M, et al. Hemochromatosis C282Y gene mutation increases the risk of venous leg ulceration. J Vasc Surg. 2005;42:309-314.
43. Tognazzo S, Gemmati D, Pallazzo A, et al. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J Vasc Surg. 2006;44:815-819.
44. Saiki S, Sakai K, Saiki MD, et al. Varicose veins associated with CADASIL result from a novel mutation in the Notch3 gene. Neurology. 2006;67:337-339.
45. Mellor RH, Brice G, Stanton AW, et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limbs. Circulation. 2007;115:1912-1920.
46. Gordeuk VR, Sergueeva AI, Miasnikova GY, et al. Congenital disorder of oxygen sensing: association of the homozygous Chuvash polycythemia VHL mutation with thrombosis and vascular abnormalities but not tumors. Blood. 2004;103:3924- 3932.
47. Badauy CM, Gomes SS, Sant’Ana Filho M, Chies JA. Ehlers-Danlos syndrome (EDS) type IV: review of the literature. Clin Oral Investig. 2007;11:183-187.
48. Smith SE, Mullen TE, Graham D, Sims KB, Rehm HL. Norrie disease: extraocular clinical manifestations in 56 patients. Am J Med Genet A. 2012;158A:1909-1917.
49. Lee S, Lee W, Choe Y, et al. Gene expression profiles in varicose veins using complementary DNA microarray. Dermatol Surg. 2005;31:391-395.
50. Eberhardt RT, Raffetto JD. Chronic venous insufficiency. Circulation. 2005;111:2398-2409.
51. Shepherd AC, Lane TR, Davies AH. The natural progression of chronic venous disorders: an overview of available information from longitudinal studies. Phlebolymphology. 2012;19:138-147.
52. Franks PJ, Morton N, Campbell A, Moffatt CJ. Leg ulceration and ethnicity: a study in west London. Public Health. 1997;111:327-329.
53. Minniti CP, Eckman J, Sebastiani P, Steinberg MH, Ballas SK. Leg ulcers in sickle cell disease. Am J Hematol. 2010;85:831-833.
54. Bollinger A, Fagrell B. Clinical capillaroscopy, a guide to its use in clinical research and practice, 1st ed. Toronto, Canada:Hogrefe and Huber Publishers; 1990:31-52.
55. Simka M. Cellular and molecular mechanisms of venous leg ulcers development–the “puzzle” theory. Int Angiol. 2010;29:1-19.
56. Zamboni P, Izzo M, Tognazzo S, et al. The overlapping of local iron overload and HFE mutation in venous leg ulcer pathogenesis. Free Rad Biol Med. 2006;40:1869-1873.
57. Zamboni P, Gemmati D. Clinical investigations of gene polymorphisms in venous leg ulcer: a model of tissue injury and reparative process. Thromb Haemost. 2007;98:131-137.
58. Ashworth JJ, Smyth JV, Pendleton N, et al. Polymorphisms spanning the ON exon and promoter of the estrogen receptor beta (ER beta) gene ESR2 are associated with venous ulceration. Clin Genet. 2008;73:55-61.
59. Mackenzie RK, Ludlam CA, Ruckley CV, Allan PL, Burns P, Bradbury AW. The prevalence of thrombophilia in patients with chronic venous leg ulceration. J Vasc Surg. 2002;35:718- 722.
60. Maessen-Visch MB, Hamulyak K, Tazelaar DJ, Crombag NH, Neumann HA. The prevalence of Factor V Leiden mutation in patients with leg ulcers and venous inufficiency. Arch Dermatol. 1999;135:41-44.
61. Lamblin N, Bauters C, Hermant X, Lablanche JM, Helbecque N, Amouyel P. Polymorphisms in the promoter regions of the MMP2, MMP3, MMP9 and MMP12 genes as determinants of aneurysmal coronary arterial disease. J Am Coll Cardiol. 2002;40:43-48.
62. Borghese B, Chiche JD, Vernerey D, et al. Genetic polymorphisms of matrix metalloproteinase 12 and 13 are implicated in endometriosis progression. Human Reprod. 2008,23:1207-1213.
63. Wallace HJ, Vandongen YK, Stacey MC. Tumor Necrosis factor alpha gene polymorphism associated with increased susceptibility to venous ulceration. J Invest Dermatol. 2006;126:921-925.
64. Nagy N, Szolnoky G, Szabad G, et al. Tumor Necrosis factor-alpha-308 polymorphism and leg ulcerationpossible association with obesity. J Invest Dermatol. 2007;127:1768.
65. Gemmati D, Tognazzo S, Catozzi L, et al. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J Vasc Surg. 2006;44:554-562.
66. Fransen K, Fenech M, Fredrikson M, Dabrosin C, Soderkvist P. Association between ulcerative growth and hypoxia inducible factor-1 alpha polymorphisms in colorectal cancer patients. Mol Carcinog. 2006;45:833- 840.
67. Cho YS, Bae JM, Chun YS, et al. HIF-1 alpha controls keratinocyte proliferation by up-regulating p21 (WAF1/Cip1). Biochem Biophys Acta. 2008;1783:323-333.
68. Anwar MA, Georgiadis KA, Shalhoub J, Lim CS, Gohel MS, Davies AH. Review of familial, genetic, and congenital aspects of primary varicose vein disease. Circ Cardiovasc Genet. 2012;5:460-466.
69. Cui C, Liu G, Huang Y, et al. Micro RNA profiling in great saphenous vein tissues of patients with chronic venous insufficiency. Tohoku J Exp Med. 2012;228:341-350.
