Chronic pelvic pain anpelvic venous disorders: the gynecologist’s point of view

Aleksandra
Jaworucka-Kaczorowska, MD, PhD
Center of Phlebology and Aesthetic
Medicine, Center of General and
Vascular Surgery, Gynecology and
Obstetrics “Jaworuccy”, Gorzów
Wlkp, Poland
ABSTRACT
Chronic pelvic pain (CPP) is a very common symptom with multiple potential etiologies. Very often, it is the result of an overlap of several pain-generating disorders of the reproductive tract, gastrointestinal system, urological organs, musculoskeletal system, and psychoneurological system. Living with CPP carries a heavy economic and social burden. Due to the complex etiology of CPP, to achieve good treatment results, the approach should be multimodal and requires a cooperative interdisciplinary team of clinicians, including gynecologists, vascular surgeons or phlebologists, interventional radiologists, gastroenterologists, urologists, physiotherapists, and psychologists. All the potential etiologies should be checked and ruled out before establishing the treatment modality. Central pain sensitization should also be considered. Appropriate management from the first presentation can improve health-related quality of life, work productivity, and health care utilization and reduce an endless series of referrals, investigations, and inappropriate treatment. This article presents the potential etiologies of CPP with a primary focus on gynecological issues, including pelvic venous disorders (PeVDs), which are an entity on the borderline of gynecology and vascular surgery and are an increasingly recognizable pathology.
Introduction
There is no consensus on the definition of chronic pelvic pain (CPP), but it generally refers to pain with duration of at least 6 months that occurs at the anatomical pelvis, anterior abdominal wall below umbilicus, lumbosacral region of the back, and buttocks, is severe enough to cause functional disability, and requires treatment. Pain can be constant, although it does not have to occur every day to be considered chronic. It may follow a regular cycle with occurrences during menstruation (dysmenorrhea), during or after intercourse (dyspareunia), before or after eating, or while urinating.1
Based on a systematic review by the World Health Organization (WHO), the prevalence rates of CPP range from 4.0% to 43.4% according to 18 studies including 299 740 women. Among the 3 high-quality studies with representative samples, the rate of CPP was 2.1% to 24%.2 The prevalence of CPP is comparable to that of other common medical problems. A cross-sectional analysis using the UK Mediplus Primary Care database found its incidence to be similar to those of asthma, back pain, and migraine, but only approximately one-third of women with CPP seek medical care.3
Living with any chronic pain carries a heavy economic and social burden. Based on a systematic review of the cost of CPP in women, the total direct outpatient medical costs are $2.8 billion per year. Fifteen percent of women with CPP miss more than 1 hour of paid work per month, and the cost of work time lost for CPP is $555.3 million per year.4 Accurate diagnosis and effective management from the first presentation could reduce the endless series of referrals, investigations, and inappropriate treatment and could improve health-related quality of life, work productivity, and health care utilization.
Etiologies of CPP
CPP is a symptom with multiple potential etiologies and very often results from overlapping disorders of the reproductive tract, gastrointestinal system, urological organs, musculoskeletal system, and psychoneurological system, which each contribute to pain. Therefore, the aim of assessment should be to identify contributory factors rather than assign causality to a single pathology. It is often impossible to identify the cause of the pain confidently at the initial assessment.1 The Royal College of Obstetricians and Gynecologists (RCOG) has divided the etiological factors causing pain into gynecological and extra-gynecological factors (Table I). The 5 most common etiologies of CPP include irritable bowel syndrome, musculoskeletal pelvic floor pain, gynecological disorders known as chronic uterine pain disorders, painful bladder syndrome, and peripheral neuropathy.1,5
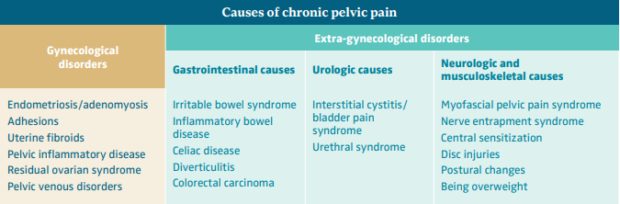
Table I. Causes of chronic pelvic pain.
Gynecological disorders
Gynecological disorders account for approximately 20% of cases of CPP.3 Despite this, 40% of laparoscopies and 12% of hysterectomies are still performed annually for CPP.6
Endometriosis and adenomyosis
Endometriosis and adenomyosis are the most common gynecological causes of CPP (Figure 1). Studies report that 20% to 80% of women who undergo surgery because of CPP are diagnosed with endometriosis. Adenomyosis has been reported to be present in approximately 20% of women undergoing surgery for endometriosis; it is most commonly seen in women with deeply infiltrative endometriosis.7 The symptoms associated with a hormonally driven condition such as endometriosis or adenomyosis include pain in the lower abdomen or pelvis, which varies markedly throughout the menstrual cycle and worsens during menstruation, hindering normal activities. It may also involve pain during or after intercourse, urination, or defecation, as well as nausea, constipation, diarrhea, and blood in urine or stool, especially during periods.
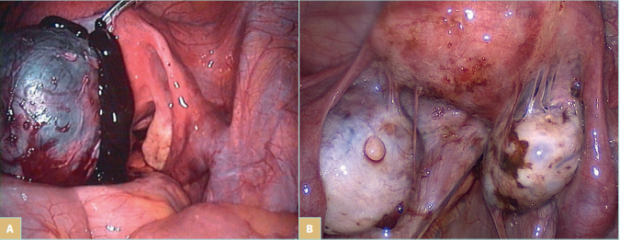
Figure 1. Laparoscopic view of endometriosis. A) An endometrial cyst of the ovary, known as “chocolate cyst.” B) Endometrial
lesions on the ovaries, uterus, and in the pelvic wall, and endometrial adhesions in the lower pelvis.
CPP in patients with endometriosis may result from significantly higher levels of antiendometrial antibodies (AEAs), regulatory T lymphocytes (Tregs), nerve growth factor (NGF), and vascular endothelial growth factor (VEGF) in the area affected by endometriosis, causing the ingrowth of nerve fibers toward the endometrial ectopic foci and neoangiogenesis in nerves.8,9 Moreover, findings demonstrate that women with endometriosis have higher levels of inflammatory factors in the peritoneal fluid and blood serum, which may also affect the severity of pain sensation.9 Pain may be due to nociceptive, neuropathic, or inflammatory mechanisms, and all 3 of these mechanisms are probably relevant to endometriosis-associated pelvic pain. However, it should be highlighted that there is no correlation between the location of disease and the location of pain. Moreover, the presence and severity of endometriosis do not consistently correlate with the severity of symptoms.9,10
Endometriosis was also found in almost 10% of asymptomatic women, whereas adenomyosis was found in 47% of hysterectomized specimens from women undergoing perimenopausal transition. However, it was defined as an incidental finding and not the source of the symptomatology.11 The coexistence of other pain-generating disorders in women with endometriosis is higher than in the general population, so it is important to treat endometriosis in symptomatic patients, as well as to diligently identify and treat all other possible sources of pain, regardless of the presence of endometriosis. Other gynecological disorders that should be taken into consideration in the differential diagnosis of CPP include adhesions, uterine fibroids, pelvic inflammatory disease (PID), residual ovary syndrome, and ovarian tumors, although CPP is not a basic or characteristic symptom of these disorders.1
Adhesions
The mechanisms of adhesiogenesis are not well understood but probably involve mesothelial surface disruption with subsequent fibrinocoagulative and inflammatory signaling processes. Etiologically, adhesions can generally be classified as post-operative, post-inflammatory, and post-radiation adhesions.12 Post-operative peritoneal adhesions have been reported to develop after more than 90% of abdominal surgeries (general, vascular, gynecological, and urological).13 As adhesions can limit organ mobility, they may cause visceral pain, particularly upon organ distension or stretching. However, adhesions may also be asymptomatic.
Uterine fibroids / uterine myoma
Uterine fibroids are a common disease in women of child bearing age. A population-based cross-sectional study including 635 participants found uterine fibroids in 15% of women (Figure 2). Women with fibroids were more likely to report moderate or severe noncyclic CPP (adjusted odds ratio [OR], 2.6; 95% confidence interval [CI], 0.9-7.6; statistically significant trend) and moderate or severe dyspareunia (adjusted OR, 2.8; 95% CI, 0.9-8.3; statistically significant trend) than women without fibroids. The number and total volume of fibroids were not related to pain.14
An international internet-based survey of 21 746 women also found that women with diagnosed uterine fibroids had the following pain symptoms significantly more often than women without uterine fibroids: CPP (14.5% vs 2.9%), painful sexual intercourse (23.5% vs 9.1%), pain occurring mid cycle, after, and during menstrual bleeding (31.3%, 16.7%, 59.7%, vs 17.1%, 6.4%, 52.0%), and pressure on the bladder (32.6% vs 15.0%). Of the women diagnosed with uterine fibroids, 53.7% reported that their symptoms had a negative impact on their life in the last 12 months, influencing their sexual life (42.9%), performance at work (27.7%), and relationship with family (27.2%).15 However, these data were based on self-report, and it is unknown whether other causes of pain were also present.
Pelvic inflammatory disease
PID refers to acute and subclinical infection of the upper genital tract in women. The prevalence rates of PID range from approximately 3% to 10%. Around 30% of women with PID subsequently develop CPP as a long-term sequela of infection or as a result of chronic subclinical infection. Patients with severe adhesive disease and tubal damage and/or persistent pelvic tenderness 30 days after diagnosis and pharmacological treatment of PID have a significantly higher risk for developing CPP.16
Residual ovarian syndrome
Residual ovarian syndrome (ROS) is a less common cause of CPP. It is a complication that occurs after hysterectomy in which one or both ovaries have been preserved and cause CPP (71%–77%) or dyspareunia (67%). The incidence of ROS is 2% to 3%, and almost 50% of patients with ROS require surgery for treatment of CPP due to ROS within the first 5 years after hysterectomy, whereas 75% require it within 10 years.17
Pelvic venous disorders
Pelvic venous disorders (PeVDs) are an entity on the borderline of gynecology and vascular surgery and are an increasingly recognizable pathology. It has been reported as a possible cause of pain in 16% to as much as 31% of women with CPP.1,2 PeVDs result from pelvic venous incompetence (PVI), which originates from the left or right gonadal vein, the left or right internal iliac vein (IIV), or a combination of these veins. PVI may be a primary incompetence or secondary to extrinsic compression or intraluminal changes (post-thrombotic iliac obstruction). The most common compression syndromes include compression of the left common iliac vein (CIV) by the right common iliac artery, known as May-Thurner syndrome; and the compression of the left renal vein (LRV) between the aorta and the superior mesenteric artery, known as Nutcracker syndrome. Extrinsic compression may also be caused by endometriosis or a tumor mass.18 Pelvic venous reflux may cause pelvic venous hypertension and subsequently varicose veins (VVs) in 3 reservoirs: the renal hilum, the venous plexuses of the pelvis (Figure 3), and the pelvic origin extra pelvic veins when the reflux is transmitted through the pelvic escape points to the veins of vulva or lower limb.18 It is worth mentioning that the presence of hypogastric vein tributaries is not necessarily correlated with pelvic reflux.
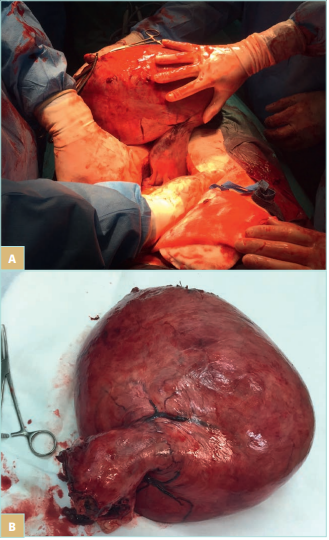
Figure 2. Myomatous uterus during hysterectomy (A, B).
It should be highlighted that most patients with PVI are asymptomatic and do not require any diagnostic and treatment at all.18,19 Some patients may develop the following symptoms related to either VVs or increased venous pressure: CPP, including dyspareunia or prolonged post-coital pain, leg edema, and/or venous claudication, venous leg ulcer (in cases of CIV compression), left flank pain, and/or hematuria (in cases of LRV compression).18 It is still unknown which patients will develop symptoms and why, whether the symptoms of the patient are related to PVI, or whether PVI is only an asymptomatic comorbidity.
Vein dilation and venous reflux are not enough to lead to both the symptoms and the diagnosis of PeVDs. There are no validated criteria or cutoff points to diagnose this disorder. Incompetent and dilated ovarian veins can be found in almost 50% of asymptomatic women, as can pelvic VVs, especially after a second pregnancy. Additionally, 90% of patients do not have valves in IIV. Asymptomatic compression of the CIV and the LRV causing ≥50% area reduction may be present in 25% to 33% and 51% to 72% of the general population, respectively.18
Hormonal factors may play a critical role in the pathophysiology and symptomatology of PeVDs. The suggestion that endogenous hormone levels matter arose from the worsening of symptoms during menstruation, an increased prevalence of PeVDs in multiparous and premenopausal women, the resolution of symptoms after menopause, and the positive therapeutic effects of hormonal substitution.20 Due to congestion and the resulting ovary overstimulation, there is an increased level of estrogen. In combination with insufficient levels of progesterone-like hormones, these conditions can cause some changes in women’s physiology and impact CPP related to PVI.20
Patients with PVI have been found to have significantly higher levels of estradiol in blood refluxing from the pelvis to the groin than in the upper extremities.21 Higher levels of estrogen may be the reason for the significantly larger uterus and thicker endometrium in patients with congestion than in healthy women.22 The mean endometrial thickness was found to be 9.9±1.8 mm in a group with pelvic VVs and was significantly higher (P=0.048) than in the healthy group, where the endometrial thickness was measured to be 6.2±2 mm.23 Hormonal imbalance may also be responsible for polycystic ovary changes in as much as 56% of women with PVI.20 According to Park et al, polycystic ovaries were found in 40.6% of patients with pelvic congestion and in 11.4% of a control group during ultrasound examination.24
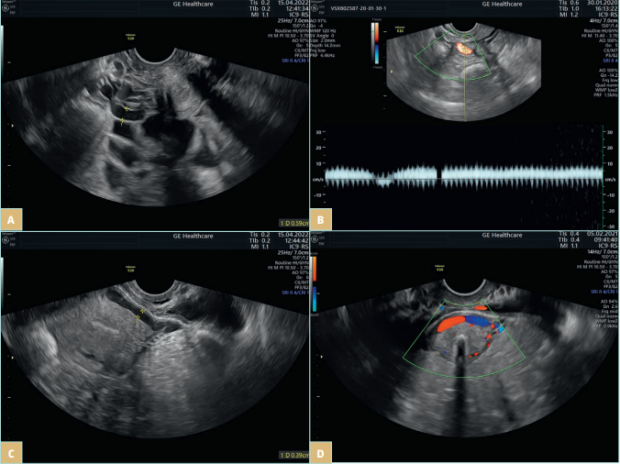
Figure 3. Transvaginal ultrasonography of a patient with a pelvic venous disorder. A) Pelvic varicocele. B) Venous reflux in periuterine venous
plexus. C-D) An arcuate vein crossing the uterine body.
Although the pathophysiology of CPP in patients with PeVDs is not fully understood, it is thought to be caused by a few estrogen-related mechanisms. Estrogen is a potent vasodilator, and its receptors are present on human vascular cells. Elevated estrogen can increase levels and activity of matrix metalloproteinases (MMPs), which cause degradation of extracellular matrix (ECM) proteins such as collagen and elastin, further decrease vein contraction, increase venous dilation, and consequently accelerate VV formation.
MMP-induced ECM degradation may also cause valve degeneration, leading to further increases in venous pressure. Increased MMPs leads to nitric oxide production, which not only weakens and dilates the uterine vessels, but also causes pelvic pain.25 Fluctuations in estradiol levels also have an effect on nociceptive sensitivity.26 Other likely mechanisms for pain in patients with PeVDs include stasis of engorged pelvic veins, which activates nociceptors within the venous wall, release of neurotransmitters from dilated pelvic veins, and compression from contiguous anatomical structures to nearby nerves.27
CPP of venous origin is often characterized as dull unilateral or bilateral pain with occasional sharp flares. Bimanual examination demonstrates more diffuse tenderness of the uterus, adnexa, parametrium, and ovarian point (the junction of the upper and middle thirds of a line drawn from the umbilicus to the anterior superior iliac spine), with no pelvic floor tenderness. Uterine and adnexal palpation usually leads to deep genital pain, whereas palpation of the ovarian point leads to pelvic pain. Symptoms are often worse with activities such as walking and prolonged standing and improve when lying down.
Although deep dyspareunia is common among women with pelvic pain from a variety of causes, pain of venous origin is more likely to be associated with prolonged postcoital ache. The combination of postcoital ache and tenderness over the ovarian point has been reported to be 94% sensitive and 77% specific for distinguishing a venous origin from other causes of pelvic pain.28 Due to the high prevalence of PVI, which is usually asymptomatic and frequently coexists with other pain-generating diseases, all possible causes of CPP need to be ruled out before deciding to treat PVI.
Extra-gynecological disorders
Extra-gynecological disorders are a common cause of CPP. In a cohort analysis of a primary care database, irritable bowel syndrome (IBS) and interstitial cystitis (IC) were the most common diagnoses of women with CPP across all age groups.3 These conditions may be primary causes of CPP, components of CPP, or secondary effects caused by efferent neurological dysfunction in the presence of chronic pain.
Irritable bowel syndrome
IBS is a gastrointestinal pain syndrome characterized by chronic or intermittent abdominal pain with variable intensity and periodic exacerbations associated with altered bowel function in the absence of any organic cause. Approximately 10% to 15% of the general population has symptoms compatible with IBS. It is diagnosed more than twice as often in women than in men. Based on a survey of 798 new referrals to a gynecological clinic, the prevalence of IBS was 37.3% compared with 27.7% among controls who visited an ear, nose, and throat (ENT) or dermatological clinic (P=0.003). Approximately 50% of women referred with CPP, dyspareunia, and dysmenorrhea had symptoms compatible with IBS (P=0.005).29
Another survey of 2304 patients found that half the women with CPP also had genitourinary symptoms, IBS, or both. IBS and stress were the most common diagnoses received by patients with CPP.3 Unfortunately, approximately 40% to 50% of individuals who meet the diagnostic criteria for IBS do not have a formal diagnosis and appropriate treatment.30 IBS is also very often a comorbidity and has high prevalence in women with endometriosis and other CPP-generating disorders, which may have a negative impact on diagnostic and treatment processes.
Interstitial cystitis/ bladder pain syndrome
IC is a diagnosis that applies to patients with chronic bladder and urinary urgency in the absence of an identifiable etiology. Little is known about the pathogenesis of IC. There is no clear evidence that inflammation or abnormalities in the interstitium of the bladder are involved.1 Recent data suggest that IC is probably one of the most common causes of CPP. IC was diagnosed in 38% to 84% of women with CPP receiving secondary care.31,32
The most common symptom of IC is pain localized in the suprapubic, pubic, vaginal, and genital areas. Some patients report unilateral lower abdominal pain or low back pain with bladder filling. Pain is usually described as intermittent, regardless of the pain site, with moderate intensity.33 Other symptoms of IC include urinary urgency, daytime frequency, dysuria, and nocturia.34 Symptoms may be triggered or exacerbated by vaginal intercourse, exercise, prolonged sitting, intake of certain foods or drinks, stress, or the luteal phase of the menstrual cycle.35,36 IC often coexists with other CPP syndromes, especially IBS, fibromyalgia, and vulvodynia.
Myofascial pelvic pain syndrome
Myofascial pelvic pain syndrome (MPPS) results from dysfunction, spasticity, or hypersensitivity of the muscles, fascia, or joints in the abdominal wall, pelvic floor, or lower back. It is an extremely common but underrecognized cause of CPP in women.37 MPPS originates at the myofascial trigger points (MTrPts), which are hyperirritable spots, usually within a taut band of skeletal muscle, that are painful upon compression and can give rise to characteristic referred pain. Trigger points can be active, causing spontaneous or latent pain. It may remain asymptomatic for years and may be activated by physical trauma, a painful event in the area, or emotional stress.38
Pain can also involve the vulva, perineum, rectum, bladder, and more distant areas such as the thighs, buttocks, or lower abdomen. It may influence urinary, bowel, and sexual function. Irritative symptoms, including vulvar or vaginal burning or itching, pain during or after intercourse, urinary urgency, frequency, and dysuria, can be reported even more frequently than CPP.39 The estimates of the prevalence of MPPS in the general population vary from 14% to 78%. It often coexists with other causes of pelvic pain. MTrPts have been identified in as much as 85% of patients suffering from urological, colorectal, and gynecological pelvic pain syndromes and can be responsible for some, if not all, symptoms related to these syndromes.40 A routine assessment for MPPS should be considered for all patients presenting for evaluation of CPP.
Nerve injury or entrapment
Surgical injury or entrapment of abdominal or pelvic nerves, such as iliohypogastric, ilioinguinal, genitofemoral, lateral femoral cutaneous, or pudendal nerves, may cause subsequent CPP in the anatomic distribution of this nerve. The incidence of nerve injury following pelvic surgery is approximately 2%. Longitudinal incisions are associated with a low risk of nerve injury. Analysis of 690 patients after low transverse Pfannenstiel incision for cesarean delivery or abdominal hysterectomy showed that moderate or severe CPP associated with nerve injury or entrapment was reported by 7% and 8.9% of women, respectively.41 This cause of CPP should especially be taken into consideration in patients with previous surgical treatment.
Central sensitization
Centralization of pain occurs when sensory pain information is abnormally processed in the central nervous system, causing central pain sensitization. This causes pain that is perpetuated by the central nervous system. It is described as a dysfunctional pain syndrome and is recognized as a systemic disease.42 There is evidence that women with CPP have decreased thresholds to pain and that CPP is often present without an obvious cause according to imaging studies, laboratory values, or physical exams. Furthermore, it may persist despite treatment of the presumed etiologies.
Women with CPP often present with several seemingly unrelated symptoms. This can be explained by coexisting CPP syndromes occurring in the same patient. Central sensitization (CS) has been demonstrated in all of these syndromes, so it provides one possible explanation for CPP. In an observational cross-sectional study, CS was found in 75% of 111 women with CPP. Patients with CS were more likely to experience pain for more than 2 years (OR, 4.98; 95% CI, 1.94-12.82; P=0.001) and other pain symptoms involving the bladder (OR, 9.87; 95% CI, 2.52-38.67; P=0.001), bowel (OR, 3.13; 95% CI, 1.31-7.48; P=0.01), back (OR, 4.17; 95% CI, 1.66-10.51; P=0.002), and vulva (OR, 3.61; 95% CI, 1.21- 10.82; P=0.02). They also had higher previous diagnoses of a mental health disorder (OR, 3.5; 95% CI, 1.5-8.4; P=0.005) or IBS (OR, 8.9; 95% CI, 1.6-49.1; P=0.01).43 Patients with CS experience more prolonged and complex pain, which may have a negative impact on the treatment outcome.
Conclusions
Due to the complex etiology of CPP and the common coexistence of several pain generating disorders, there is a necessity for more precise diagnosis by a specially trained team of experts. The approach to CPP patients should be multimodal and requires the cooperation of gynecologists, vascular surgeons or phlebologists, interventional radiologists, gastroenterologists, urologists, physiotherapists, and psychologists. It is recommended that a diagnostic protocol be established to check and rule out all the potential etiologies before treatment is determined. Patient involvement, shared decision-making, and discussion of expectations for long-term care are important parts of the evaluation process. Such an approach gives the most beneficial effects.
References
1. As-Sanie S. Chronic pelvic pain in non pregnant adult females: causes. UpToDate. Updated Feb 07, 2023. Accessed February 11, 2023. https://www.uptodate. com/contents/chronic-pelvic-pain-in nonpregnant-adult-females-causes
2. Latthe P, Latthe M, Say L, et al. WHO systematic review of prevalence of chronic pelvic pain: a neglected reproductive health morbidity. BMC Public Health. 2006;6:177.
3. Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Patterns of diagnosis and referral in women consulting for chronic pelvic pain in UK primary care. Br J Obstet Gynaecol. 1999;106(11):1156-1161.
4. Huang G, Le AL, Goddard Y, et al. A systematic review of the cost of chronic pelvic pain in women. J Obstet Gynaecol Can. 2022;44(3):286-293.e3.
5. Royal College of Obstetricians and Gynecologists. The Initial Management of Chronic Pelvic Pain: Green-top Guideline No. 41. 2012. Published May 2012. Accessed February 11, 2023. https:// www.rcog.org.uk/media/muab2gj2/ gtg_41.pdf
6. Lamvu G, Carrillo J, Ouyang C, Rapkin A. Chronic pelvic pain in women: a review. JAMA. 2021;325(23):2381-2391.
7. Leyendecker G, Bilgicyildirim A, Inacker M, et al. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto traumatisation. An MRI study. Arch Gynecol Obstet. 2015;291(4): 917-932.
8. Randall GW, Gantt PA, Poe-Zeigler RL, et al. Serum antiendometrial antibodies and diagnosis of endometriosis. Am J Reprod Immunol. 2007;58: 374-382.
9. Triolo O, Laganà AS, Sturlese E. Chronic pelvic pain in endometriosis: an overview. J Clin Med Res. 2013;5(3):153-163.
10. Kor E, Mostafavi SRS, Mazhin ZA, et al. Relationship between the severity of endometriosis symptoms (dyspareunia, dysmenorrhea and chronic pelvic pain) and the spread of the disease on ultrasound. BMC Res Notes. 2020;13(1):546.
11. Weiss G, Maseelall P, Schott LL, Brockwell SE, Schocken M, Johnston JM. Adenomyosis a variant, not a disease? Evidence from hysterectomized menopausal women in the Study of Women’s Health Across the Nation (SWAN). Fertil Steril. 2009;91(1):201- 206.
12. Tabibian N, Swehli E, Boyd A, Umbreen A, Tabibian JH. Abdominal adhesions: a practical review of an often overlooked entity. Ann Med Surg (Lond). 2017;15:9-13.
13. ten Broek RP, Strik C, Issa Y, Bleichrodt RP, van Goor H. Adhesiolysis-related morbidity in abdominal surgery. Ann Surg. 2013;258(1):98-106.
14. Lippman SA, Warner M, Samuels S, Olive D, Vercellini P, Eskenazi B. Uterine fibroids and gynecologic pain symptoms in a population-based study. Fertil Steril. 2003;80(6):1488-1494.
15. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012;12:6.
16. Trautmann GM, Kip KE, Richter HE, et al. Do short-term markers of treatment efficacy predict long-term sequelae of pelvic inflammatory disease? Am J Obstet Gynecol. 2008;198(1):30.e1-e7.
17. Dekel A, Efrat Z, Orvieto R, et al. The residual ovary syndrome: a 20-year experience. Eur J Obstet Gynecol Reprod Biol. 1996;68(1-2):159-164.
18. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
19. Khilnani NM, Meissner MH, Learman LA, et al. Research priorities in pelvic venous disorders in women: recommendations from a multidisciplinary research consensus panel. J Vasc Interv Radiol. 2019;30:781-789.
20. Jaworucka-Kaczorowska A. Conservative treatment of pelvic venous disease. Turk J Vasc Surg. 2021;30(1):S37-S43.
21. Asciutto G, Mumme A, Asciutto KC, Geier B. Oestradiol levels in varicose vein blood of patients with and without pelvic vein incompetence (PVI): diagnostic implications. Eur J Vasc Endovasc Surg. 2010;40:117-121.
22. Adams J, Reginald PW, Franks S, Wadsworth J, Beard RW. Uterine size and endometrial thickness and the significance of cystic ovaries in women with pelvic pain due to congestion. Br J Obstet Gynaecol. 1990;97(7):583-587.
23. Bora A, Avcu S, Arslan H, Adali E, Bulut MD. The relation between pelvic varicose veins and lower extremity venous insufficiency in women with chronic pelvic pain. JBR– BTR. 2012;95:215-221.
24. Park SJ, Lim JW, Ko YT, et al. Diagnosis of pelvic congestion syndrome using transabdominal and transvaginal sonography. AJR Am J Roentgenol. 2004;182:683-688.
25. MacColl E, Khalil RA. Matrix metalloproteinases as regulators of vein structure and function: implications in chronic venous disease. J Pharmacol Exp Ther. 2015;355(3):410-428.
26. Hassan S, Muere A, Einstein G. Ovarian hormones and chronic pain: a comprehensive review. Pain. 2014;155(12):2448-2460.
27. Phillips D, Deipolyi AR, Hesketh RL, Midia M, Oklu R. Pelvic congestion syndrome: etiology of pain, diagnosis, and clinical management. J Vasc Interv Radiol. 2014;25(5):725-733.
28. Meissner MH, Khilnani NM, Labropoulos N, et al. The symptoms-varices pathophysiology classification of pelvic venous disorders: a report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. J Vasc Surg Venous Lymphat Disord. 2021;9(3):568-584.
29. Prior A, Wilson K, Whorwell PJ, Faragher EB. Irritable bowel syndrome in the gynecological clinic. Survey of 798 new referrals. Dig Dis Sci. 1989;34:1820-1824.
30. Sayuk GS, Wolf R, Chang L. Comparison of symptoms, healthcare utilization, and treatment in diagnosed and undiagnosed individuals with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2017;112(6):892-899.
31. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100(2):337-341.
32. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395-1400.
33. FitzGerald MP, Brensinger C, Brubaker L, Propert K. What is the pain of interstitial cystitis like? Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(1):69-72.
34. Teichman JM, Parsons CL. Contemporary clinical presentation of interstitial cystitis. Urology. 2007;69(4):41-47.
35. Friedlander JI, Shorter B, Moldwin RM. Diet and its role in interstitial cystitis/bladder pain syndrome (IC/BPS) and comorbid conditions. BJU Int. 2012;109(11):1584- 1591.
36. Powell-Boone T, Ness TJ, Cannon R, Lloyd LK, Weigent DA, Fillingim RB. Menstrual cycle affects bladder pain sensation in subjects with interstitial cystitis. J Urol. 2005;174(5):1832-1836.
37. Billecocq S, Bo K, Dumoulin C, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for the conservative and non-pharmacological management of female pelvic floor dysfunction. Article in French. Prog Urol. 2019;29(4):183-208.
38. Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial trigger points then and now: a historical and scientific perspective. PM R. 2015;7(7):746-761.
39. Meister MR, Sutcliffe S, Badu A, Ghetti C, Lowder JL. Pelvic floor myofascial pain severity and pelvic floor disorder symptom bother: is there a correlation? Am J Obstet Gynecol. 2019;221(3):235.e1-235.e15.
40. Moldwin RM, Fariello JY. Myofascial trigger points of the pelvic floor: associations with urological pain syndromes and treatment strategies including injection therapy. Curr Urol Rep. 2013;14(5):409-417.
41. Loos MJ, Scheltinga MR, Mulders LG, Roumen RM. The Pfannenstiel incision as a source of chronic pain. Obstet Gynecol. 2008;111(4):839-846.
42. Hoffman D. Central and peripheral pain generators in women with chronic pelvic pain: patient centered assessment and treatment. Curr Rheumatol Rev. 2015;11(2):146-166.
43. Ryan A, Healey M, Cheng C, Dior U, Reddington C. Central sensitisation in pelvic pain: a cohort study. Aust N Z J Obstet Gynaecol. 2022;62(6):868-874.
