Anticoagulation and interventional treatment of varicose veins
Saint Charles Clinic, Interventional
Phlebology Unit, La Roche-sur-Yon,
France
Abstract
Venous thromboembolic risk is very low after varicose vein procedures. This risk is often cited as less than 1%; however, studies also show the risk to be highly variable. Overall, literature in the field does not typically conclude thromboprophylaxis to be necessary in low-risk patients, owing to the low incidence of the event studied and the often-insufficient number of patients included. Despite the low incidence, venous thromboembolic risk is important in terms of mortality and morbidity. Varicose veins affect an average of 1 in 3 individuals, and there is still great variability in practices concerning thromboprophylaxis throughout the world. The parallel must also be considered with regard to interventional treatment of varicose veins in patients already receiving anticoagulant therapy. Endovenous treatment of varicose veins of the lower limbs has taken precedence over conventional surgery more or less rapidly depending on the country and the level of health care reimbursement. Recommendations advocate for an endovenous rather than a surgical approach whenever possible. However, questions remain unanswered, and a standardization of practices through clear recommendations needs to be drawn.
Overview of the different interventional treatments for varicose veins and their recommendations
Open surgery is the oldest interventional treatment technique for varicose veins and remains in the phlebology practice in many countries.
Thermal endovenous treatment techniques have been developed over the last 2 decades with the advent of ultrasound, which has allowed a better understanding of venous anatomy and hemodynamics. Overall, there are 3 types of endovenous techniques (Table I): (i) tumescent thermal ablations, including endovenous laser treatment (EVLT) and radiofrequency ablation (RFA), which are the most widely used and best-studied techniques; (ii) nonthermal, nontumescent ablations, of which sclerotherapy, and more particularly ultrasound-guided foam sclerotherapy (UGFS), often also classified as “chemical ablation,” is the most widely practiced and the technique with the greatest recoil; and finally, (iii) combined ablations, a combination of thermal and nonthermal techniques, such as the treatment of a saphenous trunk by laser/RFA, and the treatment of tributaries by echosclerotherapy.1,2 All these procedures can be used with tumescent and nontumescent local anesthesia.
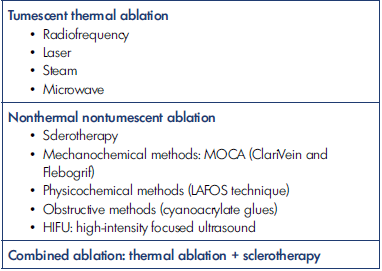
Table I. Classification and overview of the different techniques for endovenous treatment of varicose veins. LAFOS, laser-assisted foam sclerotherapy; MOCA, mechanical occlusion chemically assisted ablation
So-called “conventional” varicose vein surgery has remained the gold standard of varicose vein treatment for decades. The concept of “modern surgery” recognized in 20143 could in 2021 be summarized as the combination of minimally invasive treatment of the saphenous trunk and incompetent tributaries, with stripping being replaced by laser or RFA. Most often, crossectomy or flush ligation is abandoned.
The European Venous Forum and the International Union of Angiology3,4 recommend thermal ablation (RFA or laser), modern open surgery, or UGFS for grade 1A conditions. In 2018, the recommendations of the European Venous Forum added steam, cyanoacrylate glue, and mechanical occlusion chemically assisted ablation (MOCA) for grade 1B conditions.
The European Society of Vascular Surgery (ESVS)5 recommends endovenous thermal ablation (EVTA) as first-line interventional treatment for grade IA venous insufficiency of the great saphenous vein before surgery or UGFS.
The American Venous forum6 recommends EVTA for grade 2B classified conditions of the great saphenous vein, before UGFS and before conventional surgery.
The National Institute for Health and Care Excellence (NICE)7 recommends EVTA by RFA or EVLT as the first-line interventional treatment for the great saphenous vein. Finally, the Guidelines of the First International Consensus Conference on EVTA for varicose vein disease8 recommend EVTA for the great saphenous vein (grade 1A), the small saphenous vein (grade 1A), the accessory saphenous vein (intrafascial portion; grade 1B), and the Giacomini’s vein (grade 1B).
The “minimally invasive” nature of endovenous techniques allows them to be performed on an outpatient basis with the shortest possible immobilization of the patient, with rapid resumption of ambulation and little or no time off work. This has been well demonstrated compared with conventional surgery.9,10
Thrombotic risk
Sclerotherapy
Sclerotherapy via a sclerosing agent in liquid form has long been the reference treatment for spider veins and telangiectasias. The foam form was described during the Second World War but was really developed in the middle of the 1990s.
Development of ultrasonography then made it possible to combine injection and foam form, thus increasing the efficacy and safety of this remarkable technique, its indications being high grade and allowing the practitioner to treat all varicose veins.11 The thrombotic risk is described as low: Jia in his meta-analysis reports a thrombotic risk of around 0.6%.12 Among other things, the author notes that the large volumes of the sclerosing agent injected increase the thromboembolic risk and that the thrombotic risk factors to be taken into consideration include history of venous thromboembolic disease, thrombophilia, obesity, and sedentary lifestyle. For Kulkarni,13 the risk is about 0.9%.
Thermal endovenous treatments
The thermal endovenous treatment techniques that have been evaluated in the greatest number of quantitative and qualitative studies and for which there is the greatest hindsight are laser and RFA. The long-term safety and efficacy of these techniques has already been demonstrated.14,15 The risk of thromboembolism has also been reported to be less than 1% in most studies.16-19 The Marsh study20 found rates of around 1% for endovenous laser treatment, and this was slightly lower, at 0.7%, for RFA.
Nonthermal nontumescent endovenous treatments
For mechanochemical ablation methods (MOCA: ClariVein and Flebogrif), obstructive methods (cyanoacrylate glues: VenaSeal, Variclose, VenaBlock), physicochemical methods (LAFOS technique [laser assisted foam sclerotherapy]), and high-intensity focused ultrasound (HIFU), the studies are far fewer in number and the follow-up is less important. For MOCA, the thromboembolic event rate is less than 1% and remains lower than for endovenous thermal treatments according to the recent meta-analysis by Nugroho.21 As regards VenaSeal, the 2019 French Health Authority (HAS) report shows a low rate of thromboembolic events, again less than 1%,22 and this rate is also very low in the VeClose study (VenaSeal Sapheon Closure System Pivotal Study).23
Conventional surgery
Here again, the figures for the incidence of thromboembolic events are low, on the order of 1%.24 For Sutton, there is no difference in risk between all varicose vein interventional treatment procedures, from conventional surgery to endovenous treatments.25
For all techniques, the studies carried out have often been retrospective. It is important to bear in mind that retrospective studies necessarily show fewer thromboembolic events than prospective studies because use of Doppler ultrasound is not systematic in retrospective studies and events could have been diagnosed by another team without the team that performed the procedure being informed. What is important to remember is that despite some rare and disparate data, the majority of studies report a thromboembolic event rate of around 1%.
Finally, the European Society of Vascular Surgery5 estimates the incidence of venous thromboembolic events at 0.2% to 1.3%, with no difference between endovenous techniques and conventional surgery.
With regard to endovenous treatments, practitioners need to take into account the risk of occurrence of endovenous heat-induced thrombosis (EHIT) for thermal treatments and endovenous foam-induced thrombosis (EFIT) for sclerotherapy. For example, Sufian26 found rates of EHIT after RFA treatment of around 3%, most EHITs being asymptomatic and not all of them requiring curative treatment, as noted in the recently updated recommendations of the American Venous Forum and the Society of Vascular Surgery.27 In addition to a very precise therapeutic course of action, these recommendations give a precise definition of the entities “EHIT” and “venous thrombosis”: “EHIT, any thrombus detected by ultrasound within 4 weeks of EVTA, originating from the treated vein and protruding into a deep vein. Non-EHIT venous thrombosis: deep venous thrombosis occurring in a venous segment not contiguous to the thermally ablated vein.”
There is no scientific evidence to support the use of thromboprophylaxis or venous compression to prevent EHIT occurrence.
With regard to EFITs, the study by Kulkarni13 also found a very low rate.
Recommendations
Most recommendations agree on the need to stratify thromboembolic risk according to the type of treatment and the patient’s predisposition to develop a thromboembolic event. For example, this is well described in the Caprini score, in which it is interesting to note that the presence of varicose veins is a risk factor for developing a venous thromboembolic event perioperatively. Despite this, thromboembolic risk stratification for the treatment of varicose veins is very poorly defined.
With regard to sclerotherapy, European recommendations11 indicate that anticoagulation should be proposed in patients with a history of venous thromboembolism or severe thrombophilia. For these patients, venous compression and rapid resumption of activities are recommended, as well as avoidance of injection of too-large volumes of sclerosing foam. Overweight patients or those with limited mobility should also be considered. However, no notion of severe thrombophilia is defined.
This notion can be better understood by looking at the study of Hamel-Desnos,28 which included 105 patients with thrombophilia (heterozygous Factor V, homozygous Factor V, heterozygous Factor II, elevated Factor VIII, combination of heterozygous Factor II and V mutations, combination of heterozygous Factor V and elevated Factor VIII mutation). Thromboprophylaxis was given to all patients. No thromboembolic events occurred.
A distinction must be made between patients with minor thrombophilias, those at low risk of venous thromboembolic events, and those with major thrombophilias at higher risk of thrombosis. Two major thrombophilia groups are particularly at risk: those with antithrombin III deficiency and those with antiphospholipid syndrome. Classically, for these 2 major thrombophilias, sclerotherapy should not be performed, as there is not enough experience to date. For other thrombophilias, a benefit/risk balance should always be established before considering treatment, as recommended.
The Society of Vascular Surgery and the American Venous Forum6 recommend thromboprophylaxis in patients at risk without recommending one regimen over another.
The Venous Forum and the Royal Society of Medicine published this year, in the context of the SARS-CoV-2 pandemic, recommendations concerning thromboprophylaxis of patients treated with thermal and nonthermal endovenoustreatments.29 The authors note a lack of recommendations on this subject and a disparity in practices. The authors propose a risk stratification and suggest taking into account the intermediate-risk patient, who would not necessarily have received thromboprophylaxis outside the pandemic. With regard to the low-risk patient, it was indicated that there were no arguments for using a single-dose or a short 3-day treatment. The risk factors to be taken into account are personal or family history of thromboembolism, known thrombophilia, reduced mobility, body mass index (BMI)>30, hormone therapy, active cancer, postthrombotic syndrome, and superficial venous thrombosis.
With regard to the ESVS5 and the NICE,7 they do not make any specific recommendations concerning thromboprophylaxis. The ESVS recalls that to reduce the risk of thrombosis, the patient should be treated as an outpatient, under tumescent local anesthesia, and should be ambulatory as soon as possible. The ESVS recommends assessing risk factors according to a score such as the Caprini score and specifies a history of thromboembolism, thrombophilia, obesity, immobilization, cancer, and older age as risk factors.
The guidelines of the first international consensus on EVTA of varicose veins8 do not recommend systematic thromboprophylaxis. As for ESVS, it is recommended to evaluate the thrombotic risk by the Caprini score, for example, and to take into account age over 60 years, oral contraception, hormone therapy, history of thromboembolism, severe thrombophilias, obesity, immobilization, and cancers. Some authors note that certain interventional characteristics will increase the thrombotic risk with regard to varicose vein surgery: bilateral procedure, treatment of a recurrence, treatment of a small saphenous vein, and concomitant phlebectomies.
In France, the Health Authority (HAS; Haute Autorité de Sante)30 states that “the postoperative prescription of low molecular weight heparins (LMWH) has not been the subject of a consensus among the professionals previously consulted, except for subjects considered to be ‘at risk’ for whom preventive treatment would be prescribed.”
In 2020, the French Society of Vascular Medicine (SFMV) updated its guidelines on endovenous thermal treatments.31 It proposes prophylactic anticoagulation in patients at high risk of thromboembolism: personal history of venous thromboembolism or known major thrombophilia. It proposes a therapeutic regimen with anticoagulation via direct oral anticoagulant or LMWH or fondaparinux at a preventive dose for 7 days, and it proposes to combine it with a class 2 venous compression.
What stands out today is the need to treat patients on an outpatient basis, with the shortest possible intervention time and the fastest possible return to ambulation.
Technological progress, such as the almost systematic use of the 1470-nm wavelength for endovenous laser with radial fibers, has helped minimize undesirable effects; this is no longer in question. A new wavelength has recently been commercialized: 1940 nm. This higher wavelength allows a better absorption of the energy in the water of the venous wall, allowing basic power to be decreased. We do not have enough experience with this wavelength yet, but it will be interesting to study this aspect.
With regard to the type of thromboprophylaxis, apart from the French recommendations, there is no therapeutic scheme or recommended molecule. It has been shown that direct oral anticoagulants are safe and effective compared with LMWH and fondaparinux in the same indications, particularly in orthopedic surgery.32,33 Keo34 showed that rivaroxaban (10 mg, once daily, 3 consecutive days) was as effective in preventing the occurrence of EHIT and deep-vein thrombosis as fondaparinux.
As regards venous compression, here too we do not have any recommendations concerning the treatment of varicose veins, but we can cite the guidelines for vascular surgery published in 2006,35 which indicate a benefit in relation to the risk of thromboembolism in general surgery and vascular surgery. Despite the absence of recommendations, many articles have been published on this subject and there is still considerable controversy, but this is not the subject of this article.
Disparities in practice
What is frequently observed from one country to another, but also from one team to another within the same country or even within the same health care institution, is an incredible disparity of practices concerning thromboprophylaxis. Therapeutic strategies vary greatly: from single dose to longer duration (3 days, 7 days, 10 days, etc).
As indicated by Dattani,36 for patients at low thromboembolic risk, practices vary enormously according to practitioner and patient preferences. In the randomized controlled trial (RCT) of San Noberto37 aimed at evaluating thromboprophylaxis in the context of venous surgery, although the power of the study was considered too low to conclude, the patients included were at moderate thrombotic risk and 2 groups were formed: in the first group, thromboprophylaxis for 10 days was prescribed, whereas in the second group no pharmacological treatment was administered. No thromboembolic events occurred.
There are no consistent RCTs on the subject of thromboprophylaxis, and once again, the low incidence of thromboembolic events would require a very large cohort, and given the disparities in practice, one can imagine that there might be some difficulty in acceptance, particularly in the process of randomizing patients.
However, many articles have been published that are not controlled studies. We may cite the publication by Boyle,38 which shows that in Ireland the majority of practitioners use a single dose of thromboprophylaxis. One-third of the procedures in Ireland in that publication were endovenous treatments. The most relevant thrombotic risk factors are recognized to be thrombophilia, cancer, bilateral procedures, and obesity.
Another publication by Nikolopoulos39 also provides evidence of practice in a survey of Greek vascular surgeons. What is interesting in this study is to see that half of the patients treated by open surgery and also half of those treated by endovenous treatments received thromboprophylaxis for 2 to 5 days and in 95% of cases by LMWH. The risk factors taken into account were mainly thrombophilia, history of venous thromboembolic disease, cancer, and estrogen-progesterone contraception, but bilateral procedures, older age, or duration of surgery were not taken into account.
With regard to single-dose therapy, there is no evidence to date of its efficacy; in the study by Enoch,40 thromboembolic events occurred in the group that received single-dose therapy, whereas in the group that received no thromboprophylaxis, there were no thromboembolic events. For Boyle,38 given that varicose vein surgery is known to be the most contentious area of vascular surgery, it may therefore be advisable to administer at least a routine dose periprocedure. This remains totally debatable given the lack of demonstrated efficacy but also owing to the possible downside of such an attitude: why prescribe thromboprophylaxis for a duration that is known to be ineffective rather than stratifying the risk and prescribing, as recommended, thromboprophylaxis for a longer duration?
Risk of bleeding
What about patients who are being treated with anticoagulants and for whom varicose vein interventional treatment is being considered? This case should not be underestimated, as the overall trend in long-term anticoagulation is increasing for all causes. In France, we know that more than 2% of the general population is treated with long-term anticoagulants. It is therefore important for the practitioner not to ignore this condition.
First of all, it is necessary to know for what reason(s) the patient is treated with an anticoagulant. There are 2 cases: cardiac pathologies and venous thromboembolic disease. In both cases, it is of course imperative that the benefit-risk balance of the planned treatment of varicose veins, as well as the consequences of the absence of treatment, always be raised in these fragile patients. Next, the practitioner should ask himself a few questions: for patients with a history of venous thromboembolic pathology, has a thrombophilia screening been performed? As we have seen previously, for certain thrombophilias, even in a patient who is anticoagulated, sclerotherapy is not indicated. Another very important point to take into account in these patients, who have already had 1 or more venous thromboses, is the possible presence of obstructive or occlusive sequelae of the deep venous network and the possible need to preserve suppleance veins.
In this field, the literature provides us with some information. The first series on this subject date back to the 1980s with Dastain41 and then Franchitti42 who, in 2 small series, demonstrated that sclerotherapy in patients with long-term anticoagulation was safe and effective, with no significant difference in terms of efficacy compared with patients without anticoagulation. Another publication from 2002 by Gachet43 showed, in longterm anticoagulated patients, that sclerotherapy is safe, but the author suggests it takes more sclerotherapy sessions to bring about venous occlusion than in nonanticoagulated patients. This is also the conclusion of a study by Stücker.44
In 2009, Darvall45 published a study of 27 patients who underwent sclerotherapy for venous ulcer treatment. Four of these patients were treated with warfarin. No difference in efficacy or safety was shown in these patients. Hager in 201646 published a study whose objective was to highlight the factors influencing the occlusion of incontinent perforating veins according to 3 treatment modalities: UGFS, RFA, and EVLT. In each of these 3 groups, one-third of the patients were on long-term anticoagulants. It was shown that anticoagulation was not a predictor of failure.
Sharifi47 also studied the effect of anticoagulation in the context of thermal endovenous treatment of the great saphenous vein. This was also a small series, and no significant difference was found between the groups studied.
Takahashi’s team48 also published a study including 1136 patients who received thermal endovenous treatment of the great saphenous vein or the small saphenous vein. Of the patients included, 12% had antiplatelet aggregation treatment and 8% were receiving anticoagulation treatment. There was no significant difference between the different groups of patients regarding the rate of recanalization and postoperative complications.
A study by Theivacumar49 published in 2009 shows that warfarin does not influence the success of endovenous treatment by laser of the great saphenous vein.
Finally, Sufian50 did not find more bleeding when evaluating RFA procedures in anticoagulated patients and showed a slight decrease in the incidence of EHITs and an increase in the incidence of treatment failure in these patients.
Finally, it should be noted that the European guidelines11 for sclerotherapy report that anticoagulation is not a contraindication to the practice of sclerotherapy.
What emerges from these different studies is that there is no difference in effectiveness and safety. Of course, these are small series, uncontrolled studies.
The practitioner should remember that there is no need to increase in the first instance, for example, the concentration of the sclerosing agent in a patient treated with an anticoagulant for fear of a decrease in its effectiveness. We must continue to follow the recommendations in terms of therapeutic procedures, whatever they may be, and not forget that the indication for the treatment of varicose veins must always be weighed on a case-by-case basis. It should also be remembered that patients with long-term anticoagulation are fragile and have other comorbidities that need to be taken into account, and that interventional approach will be more appropriate for these patients than open surgery.
Conclusion
Practices concerning thromboprophylaxis for the treatment of varicose veins remain very heterogeneous. Varicose vein procedures are still the most widely performed in the world, and there is no doubt that the increase and aging of the world’s population will only reinforce this observation.
The low incidence of venous thromboembolic events leads practitioners to doubt the usefulness of this procedure for certain patients. Endovenous treatments are overtaking open surgery more or less rapidly depending on the country concerned and for various reasons (reimbursement, habits, lack of mastery of sonography, etc).
There is no doubt that in the years to come other endovenous treatment techniques that do not require an operating area will become the rule.
The practitioner must ask himself the question of the thromboembolic risk of his patient: either the risk is present and a thromboprophylaxis of 8/10 days must be prescribed, or his patient does not have this risk and no thromboprophylaxis must be instituted. This is of course within the recommended practice conditions; the treatment of varicose veins must be as short as possible, with local anesthesia, and ambulation must be resumed as soon as possible. Under these conditions, the analysis of the thrombotic risk is simplified and remains intrinsic to the patient.
To support the practitioner, publication of national and international guidelines is needed in order to harmonize our practices, make them safer, and limit their economic impact.
REFERENCES
1. Eklof B, Perrin M, Delis KT, Rutherford RB, Gloviczki P. Updated terminology of chronic venous disorders: the VEIN-TERM transatlantic interdisciplinary consensus document. J Vasc Surg. 2009;49(2):498- 501.
2. Vasquez M, Gasparis AP. A multicenter, randomized, placebo-controlled trial of endovenous thermal ablation with or without polidocanol endovenous microfoam treatment in patients with great saphenous vein incompetence and visible varicosities. Phlebology. 2017;32(4):272- 281.
3. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. Int Angiol. 2014;33(2):87-208.
4. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines according to scientific evidence. Part II. Int Angiol. 2020;39(3):175-240.
5. Wittens C, Davies AH, Bækgaard N, et al. Editor’s Choice – Management of chronic venous disease: clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2015;49(6):678-737.
6. Gloviczki P, Comerota AJ, Dalsing MC, et al; Society for Vascular Surgery; American Venous Forum. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(5 suppl):2S-48S.
7. Marsden G, Perry M, Kelley K, Davies AH. Diagnosis and management of varicose veins in the legs: summary of NICE guidance. BMJ. 2013;347:f4279.
8. Pavlović MD, Schuller-Petrović S, et al. Guidelines of the First International Consensus Conference on Endovenous Thermal Ablation for Varicose Vein Disease – ETAV Consensus Meeting 2012. Phlebol J Venous Dis. 2015;30(4):257-273.
9. Leopardi D, Hoggan BL, Fitridge RA, Woodruff PW, Maddern GJ. Systematic review of treatments for varicose veins. Ann Vasc Surg. 2009;23(2):264-276.
10. Murad MH, Coto-Yglesias F, Zumaeta- Garcia M, et al. A systematic review and meta-analysis of the treatments of varicose veins. J Vasc Surg. 2011;53(5 suppl):49S- 65S.
11. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014; 29(6):338-354.
12. Jia X, Mowatt G, Burr JM, Cassar K, Cook J, Fraser C. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94(8):925-936.
13. Kulkarni SR, Messenger DE, Slim FJ, et al. The incidence and characterization of deep vein thrombosis following ultrasoundguided foam sclerotherapy in 1000 legs with superficial venous reflux. J Vasc Surg Venous Lymphat Disord. 2013;1(3):231- 238.
14. Hoggan BL, Cameron AL, Maddern GJ. Systematic review of endovenous laser therapy versus surgery for the treatment of saphenous varicose veins. Ann Vasc Surg. 2009; 23(2):277-287.
15. Lurie F, Creton D, Eklof B, et al. Prospective randomized study of endovenous radiofrequency obliteration (closure procedure) versus ligation and stripping in a selected patient population (EVOLVeS Study). J Vasc Surg. 2003;38(2):207-214.
16. Van der Velden SK, Biemans AM, De Maeseneer MGR, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. Br J Surg. 2015;102(10):1184-1194.
17. Rasmussen L, Lawaetz M, Bjoern L, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg. 2013;58(2):421-426.
18. Carradice D, Mekako AI, Mazari FK, Samuel N, Hatfield J, Chetter IC. Clinical and technical outcomes from a randomized clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg. 2011;98(8):1117-1123.
19. Malgor RD, Gasparis AP, Labropoulos N. Morbidity and mortality after thermal venous ablations. Int Angiol J Int Union Angiol. 2016; 35(1):57-61.
20. Marsh P, Price BA, Holdstock J, et al. Deep vein thrombosis (DVT) after venous thermoablation techniques: rates of endovenous heat-induced thrombosis (EHIT) and classical DVT after radiofrequency and endovenous laser ablation in a single center. Eur J Vasc Endovasc Surg. 2010;40:521-527.
21. Nugroho J, Wardhana A, Ghea C. Mechanical occlusion chemically assisted ablation (MOCA) for saphenous vein insufficiency: a meta-analysis of a randomized trial. Int J Vasc Med. 2020;2020:8758905.
22. Haute Autorité de Sante (HAS), Commission Nationale d’évaluation des Dispositifs Medicaux et des Technologies de Sante. April 9, 2019. https://www.has-sante.fr/upload/docs/ application/pdf/2019-06/venaseal_23_ avril_2019_5761_avis_occultation.pdf
23. Morrison N, Kolluri R, Vasquez M, Madsen M, Jones A, Gibson K. Comparison of cyanoacrylate closure and radiofrequency ablation for the treatment of incompetent great saphenous veins: 36-month outcomes of the VeClose randomized controlled trial. Phlebology. 2019;34(6):380-390.
24. Van Rij AM, Chai J, Hill GB, et al. Incidence of deep vein thrombosis after varicose vein surgery. Br J Surg. 2004;91:1582-1585.
25. Sutton PA, El-Dhuwaib Y, Dyer J, et al. The incidence of postoperative venous thromboembolism in patients undergoing varicose vein surgery recorded in hospital episode statistics. Ann Royal Coll Surg Engl. 2012;94:481-483.
26. Sufian S, Arnez A, Labropoulos N, Lakhanpal S. Incidence, progression, and risk factors for endovenous heat-induced thrombosis after radiofrequency ablation. J Vasc Surg Venous Lymphat Disord. 2013;1(2):159-164.
27. Kabnick LS, Sadek M, Bjarnason H, et al. Classification and treatment of endothermal heat-induced thrombosis: recommendations from the American Venous Forum and the Society for Vascular Surgery. J Vasc Surg Venous Lymphat Disord. 2021;9(1):6-22.
28. Hamel-Desnos C, Gillet JL, Desnos P, Allaert FA. Sclerotherapy of varicose veins in patient with documented thrombophilia: a prospective controlled randomized study of 105 cases. Phlébologie. 2010;63(1):37-44.
29. Nyamekye IK, Campbell B. UK Royal Society of Medicine Venous Forum VTE Advice 2020. Phlebology. 2021;36(2):88- 90.
30. Haute Autorité de Sante (HAS). Transcutaneous laser occlusion of saphenous vein. Update of the evaluation conducted in 2008. 2016. https://www. has-sante.fr/jcms/c_2587776/fr/occlusionde- veine-saphene-par-laser-par-voieveineuse- transcutanee.
31. Gracia S, Miserey G, Risse J, et al. Update of the SFMV (French Society of Vascular Medicine) guidelines on the conditions and safety measures necessary for thermal ablation of the saphenous veins and proposals for unresolved issues. J Med Vasc. 2020;45(3):130-146.
32. Akin M, Schäfer A, Akin I, Widder J, Brehm M. Use of new oral anticoagulants in the treatment of venous thromboembolism and thrombotic prophylaxis. Cardiovasc Hematol Disord Drug Targets. 2015;15(2):92-96.
33. Ageno W, Mantovani LG, Haas S, et al. Safety and effectiveness of oral rivaroxaban versus standard anticoagulation for the treatment of symptomatic deep-vein thrombosis (XALIA): an international, prospective, non-interventional study. Lancet Haematol. 2016;3(1):e12-e21.
34. Keo HH, Baumann F, Diehm N, Regli C, Staub D. Rivaroxaban versus fondaparinux for thromboprophylaxis after endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2017;5(6):817-823.
35. Nicolaides AN, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thrombembolism. International consensus statement (guidelines according to scientific evidence). Int Angiol. 2006;25:101-161.
36. Dattani, N, Shalhoub, J, Nandhra, S, et al; The Vascular and Endovascular Research Network (VERN) Collaborators and Nyamekye I. Reducing the risk of venous thromboembolism following superficial endovenous treatment: a UK and republic of Ireland consensus study. Phlebology. 2020;35:706-714.
37. San Norberto Garcia EM, Merino B, Taylor JH, et al. Low-molecular-weight heparin for prevention of venous thromboembolism after varicose vein surgery in moderate-risk patients: a randomized, controlled trial. Ann Vasc Surg. 2013;27:940-946.
38. Boyle E, Reid J, O’Donnell M, Harkin D, Badger S. Thromboprophylaxis for varicose vein procedures – a national survey. Phlebology. 2019;34(9):598-603.
39. Nikolopoulos ES, Charalampidis DG, Georgakarakos EI, Georgiadis GS, Lazarides MK. Thromboprophylaxis practices following varicose vein surgery. Perspect Vasc Surg Endovasc Ther. 2012; 24(2):80-86.
40. Enoch S, Woon E, Blair SD. Thromboprophylaxis can be omitted in selected patients undergoing varicose vein surgery and hernia repair. Br J Surg. 2003;90:818-820.
41. Dastain J.Y. Sclerosis of varicose veins in patients on anticoagulants: a report on 2 patients. Phlébologie. 1981;34(1):73-76.
42. Franchitti D. Sclerotherapy in patients on long-term anticoagulant therapy for heart disease. Phlébologie. 1995;48:31-32.
43. Gachet G., Spini L. Sclerotherapy of varicose veins on anticoagulants. Phlébologie. 2002;55:41-44.
44. Stücker M, Reich S, Hermes N, Altmeyer P. Safety and efficiency of perilesional sclerotherapy in leg ulcer patients with postthrombotic syndrome and/or oral anticoagulation with phenprocoumon. J Dtsch Dermatol Ges. 2006;4(9):734-738.
45. Darvall KA, Bate GR, Adam DJ, Silverman SH, Bradbury AW. Ultrasound-guided foam sclerotherapy for the treatment of chronic venous ulceration: a preliminary study. Eur J Vasc Endovasc Surg. 2009;38(6):764-769.
46. Hager ES, Washington C, Steinmetz A, Wu T, Singh M, Dillavou E. Factors that influence perforator vein closure rates using radiofrequency ablation, laser ablation, or foam sclerotherapy. J Vasc Surg Venous Lymphat Disord. 2016;4(1):51-56.
47. Sharifi M, Mehdipour M, Bay C, Emrani F, Sharifi J. Effect of anticoagulation on endothermal ablation of the great saphenous vein. J Vasc Surg. 2011;53(1):147-149.
48. Takahashi K, Ito H, Katsube T, et al. Association between antithrombotic therapy and risk of postoperative complications among patients undergoing endovenous laser ablation. J Vasc Surg Venous Lymphat Disord. 2017;5(3):339- 345.
49. Theivacumar NS, Gough MJ. Influence of warfarin on the success of endovenous laser ablation (EVLA) of the great saphenous vein (GSV). Eur J Vasc Endovasc Surg. 2009;38(4):506-510.
50. Sufian S, Arnez A, Labropoulos N, Lakhanpal S. Endothermal venous ablation of the saphenous vein on patients who are on anticoagulation therapy. Int Angiol. 2017;36(3):268-274.

