Anatomical distribution of tissue fluid and lymph in soft tissues of lower limbs in obstructive lymphedema—hints for physiotherapy
Pradeep JAIN4
Govinda AMBUJAM4
Marzanna ZALESKA1
Marta CAKALA1
Transplantology, Medical Research Center,
Polish Academy of Sciences, Warsaw, Poland
2. Department of Gastrointestinal and
Transplantation Surgery, Central Clinical
Hospital, Ministry of Internal Affairs, Warsaw,
Poland
3. Rikshospitalet / Norwegian Radium Hospital,
Oslo, Norway
4. Indian Lymphology Centers, BHU Varanasi and
TMC Thanjavur
ABSTRACT
Knowledge of the exact location of tissue fluid (TF) and stagnant lymph (L) in lymphedema is indispensable to rational physiotherapy and specifically defines where to apply external pressure and how much. We visualized the “TF&L” space in the skin and subcutaneous tissue of the foot, calf, and thigh in various stages of lymphedema, using special staining techniques, in specimens obtained during lymphatic microsurgical procedures or tissue debulking. With the collecting trunks obliterated, L was present only in the subepidermal lymphatics, whereas the bulk of mobile TF accumulated in the spontaneously formed spaces in the subcutaneous tissue, around small veins, above and below the muscular fascia. Deformation of subcutaneous tissue by free fluid led to formation of multiple interconnecting tissue channels. Thus, massaging of tissues can propel TF through the spontaneously formed tissue channels, but not the partially or totally obliterated lymph collectors. The subepidermal lymphatic network conducts only a small fraction of L. Pneumatic compression therapy promoted formation of TF fluid channels.
INTRODUCTION
Lymphedema is a symptom of morphologically or functionally insufficient lymph transport. There are several etiological factors damaging the lymphatic pathways. Infections and trauma of limb skin and deep tissues evoke reaction of peripheral lymphatics and lymph nodes.1-4 Gradually, lymphatic structures become destroyed, tissue fluid transport toward and along lymphatics slows down, and edema of the dermis, subcutaneous tissue, as well as the muscular fascia and muscles gradually develops. Besides inflammation and trauma, the iatrogenic damaging factors for lymphatics are surgery and irradiation of lymph nodes in cancer therapy. Subsequently, changes in tissue and collecting lymphatics similar to those observed after infection and trauma develop (Figure 1).5,6 In addition, the remaining inguinal lymph nodes atrophy due to lack of antigenic stimulation by antigens in afferent lymph. The degree of edema depends on whether obstruction affects the superficial or deep lymphatic system or both. Damage to the superficial collecting trunks is followed by edema of the skin and subcutaneous tissue, whereas obstruction of both drainage systems brings about fast and difficult to control accumulation of tissue fluid not only in superficial tissues, but also under the muscular fascia and between muscle fibers.
Our image of the limb lymphatic network in physiological conditions as well as lymphedema is based on lymphograms or lymphoscintigrams depicting the superficial and deep systems and lymph nodes.7,8 These techniques do not visualize minor lymphatic structures under the epidermis where stagnant lymph accumulates. Direct lymphangiography with fluorescent tracers may help delineate minor dermal lymphatics, but is rarely used as it requires special equipment.9 Ultrasonography, computer-assisted tomography, and magnetic resonance imaging (MRI) visualize tissue spaces filled with stagnant tissue fluid, but do not show lymphatics.10-12 None of these methods provides a full view of the TF&L space. It is difficult to imagine how tissue fluid, in the areas with obstructed main lymphatics, finds its way to the normal uncongested tissue regions and is absorbed there. So far, only anatomical dissection and histological processing of biopsy material can visualize the tissue lymphatic network and the sites of accumulation of excess mobile tissue fluid.
In this study we visualized and calculated the volume of the TF&L space in skin and subcutaneous tissue of the foot, calf, and thigh in obstructive lymphedema stages III and IV in specimens obtained during lymphatic microsurgical procedures or tissue debulking. In order to follow the development of TF channels, lymphoscintigraphy and biopsies were performed in two patients before and after 100 sessions of pneumatic compression. The recorded observations provide useful hints for designing pneumatic devices and rational manual lymphatic massage to move stagnant tissue fluid toward unswollen regions.
MATERIAL AND METHODS
Tissue specimens
Groin, calf, and foot skin and subcutaneous tissue and inguinal lymph node specimens were obtained from 20 randomly selected patients with lower limb obstructive lymphedema stages III and IV, successively, as they came to our outpatient clinic for elective lymphovenous shunt or debulking surgery. Controls were specimens from 12 orthopedic patients with normal limbs operated upon for correction of fracture malunion. Fragments of inguinal lymph nodes were harvested during the lymphovenous shunt operations.
Lymphedema developed spontaneously or after an episode of dermatitis or following infected foot abrasion. At the time of admission, swelling had been present for an average of 7±1 years. Sixty percent of patients had experienced at least one attack of recurrent dermatolymphangioadenitis over the previous year and were treated with antibiotics. Staging of edema was based on our own classification.13 Briefly, stage 1 corresponds to edema of foot, pitting subsiding after rest, stage 2 to edema of foot and up to the mid calf, only partly subsiding from the foot, stage 3 to nonsubsiding edema of foot and calf, hyperkeratosis of toe skin, and stage 4 to edema of entire limb, and hard foot and calf skin. All patients underwent limb lymphoscintigraphy performed with 99Tc labeled aggregated albumin (Nanocoll, Amersham, Switzerland). No superficial collecting trunks could be visualized in any case. MRI was performed to evaluate the thickness of the subcutis and its water content. We excluded specimens from patients with acute dermatolymphangioadenitis, skin ulcers, chronic venous insufficiency, limb ischemia, lipedema, and rheumatoid arthritis. The study was approved by the ethics committee of the Warsaw Medical University and the Indian Council for Medical Research. Oral informed consent was obtained.
Soft tissue staining for visualizing the lymph and tissue fluid space
Sites of accumulation of stagnant lymph and tissue fluid in the interstitial space were visualized by injecting the composite skin, subcutaneous tissue, and fascia blocks with Paris blue dye in chloroform suspension.14,15 Fragments of lymph nodes were injected under the capsule. Large particles of this dye specifically enter lymphatics, but not blood vessels. They are retained in dilated free tissue spaces and stain their walls. The injected tissue fragments were placed in 5% formaldehyde, treated with increasing concentrations of ethyl alcohol, and made translucent using methyl salicylate solution. One to three hundred thick fragments were sectioned and investigated under the light transmission microscope. The surface area of stained structures was measured under the light microscope, magnification x100, using Olympus Microimage software (Olympus, Japan), and expressed as a percentage of the area of the microscopic field. The longitudinal and vertical lengths of stained spaces were measured to calculate their volumes and were expressed as a percentage of tissue fragment volume.
In order to prove that the stained spaces were not blood vessels, five-by-five mm thick fragments of Paris blue–injected tissues were snap frozen at 70°C and sectioned for immunohistochemical evaluation of the bluish stained structures. They were stained with monoclonal antibodies to lymphatic endothelial cell hyaluronan receptor LYVE-1 (R&D, Europe) and FVIII and CD31 (Dako, Glostrup, Denmark) to identify blood endothelial cells.
RESULTS
Lymphoscintigraphy showed in most patients lack of patent superficial and deep lymphatic collecting trunks (Figure 1) and MRI displayed a “honeycomb” structure especially close to the muscular fascia (Figure 2). Skin, subcutaneous tissue, and muscular fascia specimens obtained from these patients, stained with hematoxylineosin, monoclonal antibodies, and Paris blue showed dilatation of the subepidermal lymphatics and tissue fluid spaces in the subcutaneous tissue, around small veins, and in the muscular fascia (Figure 3).
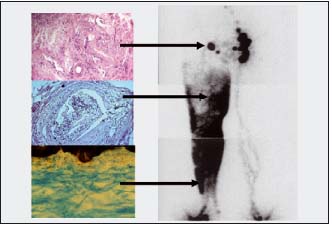
Figure 1. Lymphoscintigraphy of damaged lymphatics and nodes and histology of biopsy material of these structures in obstructive lymphedema. Right panel: a lymphoscintigram of lower limbs in a patient 5 years after acute lymphangitis. The tracer injected into toe webs spreads in the subepidermal lymphatic plexus and tissue spaces. No collecting trunks are visible. Small solitary lymph nodes in the inguinal area. Left panel: histological picture of the biopsied node shows fibrosis and irregularly shaped lymph channels (x200). Below a picture of a collecting trunk almost totally obliterated, filled with a clot with mononuclear infiltrates (H-E staining, x200). Bottom picture depicts irregular network of subepidermal lymphatics (Paris blue staining, x100)
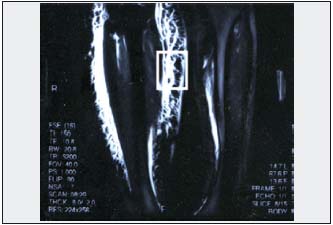
Figure 2. Magnetic resonance image of lower limb obstructive lymphedema stage III. Thickened skin and wide layer of subcutis of a honeycomb appearance. Biopsy material was taken from this region for histological evaluation of spontaneously formed “tissue channels” (frame).
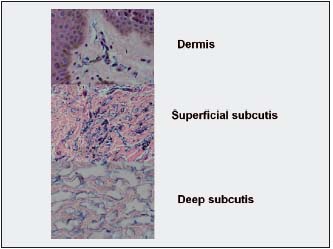
Figure 3. Histological picture of calf epidermis, dermis, and subcutaneous tissue in obstructive lymphedema stage III. Specimen stained with Paris blue and H-E. Thick epidermis composed of 10-15 layers of keratinocytes. Bluish stained minor structures in the papillary dermis are
multiple small dilated subepidermal lymphatics. In the subcutaneous tissue, bluish stained wide spaces filled with fluid are seen. Deeper in the subcutis these spaces become larger (x200).
Subepidermal lymphatic plexus
The dilated subepidermal lymphatic plexus could be easily discriminated from blood vessels by positive LYVE- 1 staining and by their shape (Figure 4). This plexus could be stereoscopically visualized by intradermal injection of Paris blue in chloroform suspension (Figures 5A,B). The venous pattern of the same skin region looked totally different from lymphatics (Figure 5D). In the course of lymphedema, the subepidermal plexus was undergoing gradual destruction, its deeper vessels becoming obstructed.
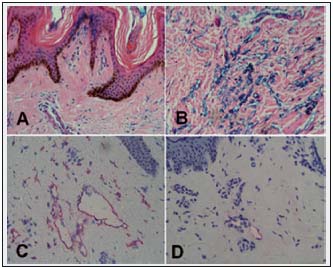
Figure 4. Subepidermal lymphatics of calf skin of the patient as in Fig. 2. A. Thick epidermis, multiple small openings in papillae stained by intravascular injection of Paris blue (x100). B. Reticular layer of skin. Multiple bluish stained spaces—minor lymphatics and tissue spaces
(x100). C. Dilated skin lymphatics stained with anti-LYVE-1 monoclonal antibody (x200). D. In other fragments they look partly obliterated, with some infiltrates (x200).
Subepidermal lymphatic plexus
The dilated subepidermal lymphatic plexus could be easily discriminated from blood vessels by positive LYVE- 1 staining and by their shape (Figure 4). This plexus could be stereoscopically visualized by intradermal injection of Paris blue in chloroform suspension (Figures 5A,B). The venous pattern of the same skin region looked totally different from lymphatics (Figure 5D). In the course of lymphedema, the subepidermal plexus was undergoing gradual destruction, its deeper vessels becoming obstructed.
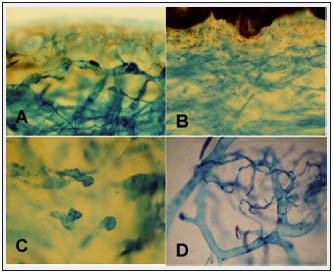
Figure 5. Skin subepidermal lymphatics stained with Paris blue in A. Venous obstruction and B,C lymphedema stage IV (x100). A. Multiple dilated lymphatics. B. Underneath epidermis a dense network of small patent lymphatics. C In reticular layer most lymphatics are obliterated. D. Retrograde injection of the dye visualized veins forming a network of small vessels merging with larger vessels. Venous architecture is different from that of lymphatics.
Subcutaneous tissue and tissue fluid channels
Most stagnant tissue fluid accumulated in the deepest layers of the subcutaneous tissue composed of fibrous and fat tissue. Excess tissue fluid deformed tissue structures leading to formation of irregularly shaped channels (Figures 6A,B). Their walls were not lined with lymphatic endothelial cells and were not stained by monoclonal antibodies against LYVE-1. With progression of lymphedema, the subcutaneous space became richer in fibrous structures, newly formed channels closed down, and fluid accumulated in narrow spaces between collagen bundles.
Perivascular spaces and muscular fascia
Mobile tissue fluid was also found in the perivascular areas (Figure 6A). Fluid accumulating in the thickened fascia formed multiple narrow longitudinal channels.
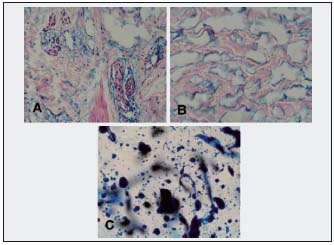
Figure 6. Calf subcutaneous tissue at the border with muscular fascia (same patient as in Fig. 2). A. Large spaces around small veins and between collagen bundles (x100). B. Large spaces are dilated artificial tissue spaces. They are not lined by lymphatic endothelial cells (LYVE-1–negative) (Paris blue staining, x100). C. Following 100 1-hour 120 mm Hg compression episodes, new channels are being formed in the subcutis. These are multiple round spaces filled with Paris blue. They look different from those in B without massage therapy. (x100).
Quantitative evaluation of TF&L volume
Quantitative evaluation of the surface and volume of dilated subepidermal lymphatics and spontaneously formed tissue spaces revealed that up to 60% of tissue volume is occupied by stagnant lymph and tissue fluid (58±6% in stage III and 32±6% in stage IV). In two stage III patients, compression therapy increased the TF&L space volume to 65±6%.
Lymph nodes
Inguinal lymph nodes revealed obliterated lymphatic sinuses (Figure 7). Their endothelial cells did not stain with antibodies against LYVE-1. No perinodal accumulation of fluid was seen.
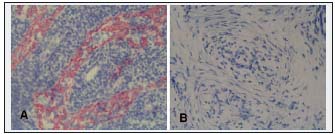
Figure 7. Histological picture of an inguinal lymph node. A. Normal appearance, red stained node lymphatic sinuses (LYVE-1–positive) (x200). B. Fibrotic node without sinuses (x200). Lymph nodes create an obstacle for lymph flow from distal parts of the extremity.
The effect of pneumatic compression therapy on tissue fluid channels
On lymphoscintigraphy, performed in similar isotope recording conditions and after 100 sequential pneumatic compression sessions of one hour each at 120 mm Hg, new TF channels could be seen reaching groin level compared with pretreatment recordings (Figure 8). Comparison of calf subcutaneous tissue biopsies stained with Paris blue revealed after treatment round spaces not observed before massage (Figure 6C).
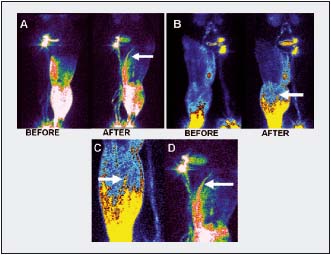
Figure 8. Lymphoscintigrams showing formation of tissue channels after long-lasting compression therapy. A. A channel not seen before treatment reaching groin area (arrow). B. Multiple channels in the calf at higher level than before treatment (arrow). C, D. Under higher magnification, the structure of new channels differs from that of lymphatics (arrows).
DISCUSSION
Knowledge of where excess limb tissue fluid and lymph accumulate is indispensable for rational physical therapy. So far, this knowledge has been based on lymphoscintigraphy, ultrasonography, and magnetic resonance imaging. None of these techniques provide combined pictures of dilated lymphatics and tissue fluid–filled spaces in the dermis, subcutis, and muscles. Only anatomical dissection and histological processing of biopsy material can show the unobstructed fragments of lymphatic network and tissue spaces—sites of accumulation of mobile fluid. In our studies we visualized the sites of accumulation of stagnant lymph and tissue fluid and determined their volume. In patients with obstructed limb lymph collectors, lymph was present only in the subepidermal lymphatics, whereas mobile tissue fluid accumulated in the spontaneously formed spaces in the subcutaneous tissue, along small veins and above and below the muscular fascia. Foot, calf, and thigh skin and subcutaneous tissue of stages IIIV lymphedematous lower limbs contained similar calculated volumes of tissue fluid reaching on average 50% of the total tissue volume (Figure 6).
The most superficial tissue layer accumulating fluid was the subepidermal lymphatic plexus in a 200-300 μ thick papillary and reticular dermis. The volume of fluid in this plexus was negligible compared with that of the subcutaneous tissue and did not exceed 2% to 3% of total fluid retained in soft tissues (data not shown). It is unclear why subepidermal lymphatics remained patent while the collecting trunks were obstructed. However, it is known that the progressive fibrosis obliterates the plexus process in stage IV. The bulk of mobile tissue fluid accumulated in the subcutaneous tissue forming artificial interconnected spaces. These spaces were located between collagen bundles and fat globules, and around small veins. Formation of large perivascular spaces by tissue fluid could be explained by the presence of lax connective tissue in these regions, its high compliance and subsequently low resistance to fluid flow.
A new finding was formation of tissue fluid channels in and around the hypertrophic muscular fascia of the calf. This was observed in patients in whom both the superficial and deep systems of limb collectors were obstructed. There were narrow longitudinal spaces between the fascia and fibrous elements. The hydraulic conductivity of these structures was presumably high because of linear positioning of fibers.
The inguinal lymph nodes revealed major changes in the sinuses as obliteration and formation of blind spaces and depletion of lymphoid elements. High resistance to lymph flow in the fibrotic nodes could be a factor causing stagnation of lymph in the rudimentary and still patent afferent lymphatics. There was no perinodal accumulation of tissue fluid.
The volume of fluid accumulating in the tissue spaces calculated from densitometric readings of slides of the stained tissues reached 50% to 60% of tissue volume. Measuring tissue fluid content and its topographical distribution may be done using noninvasive methods as MRI. However, the resolution of MRI is still too low to show minor lymphatics, small tissue fluid “lakes”, and thin fluid layers above and below fascia. Fumiere et al found that normal subcutaneous septa are seen as hyperechogenic lines in ultrasound and low signals in MRI and that hyperechogenic subcutis in ultrasound can be due to interlobular and intralobular water accumulation and/or to interlobular and intralobular fibrosis.16 They recommended multiple imaging modalities to delineate precisely the nature of tissue water accumulation in lymphedema. Idy et al demonstrated retained water diffusely spreading over the entire dermis, and fluid retention in the interlobular spacing and beside the superficial fascia. Inside the subcutis, they identified superficial fat lobules, but not much fluid accumulation.11 However, these images did not precisely depict the location and shape of tissue fluid–formed spaces and the structure of their walls. Our observations, based on microscopy studies of harvested tissue, supplement the knowledge obtained from noninvasive imaging on the topography of mobile fluid accumulation and the shape of channels in the edemadeformed tissue.
The recorded data provide hints on how to design the shape of pneumatic sleeves and where to press manually in order to effectively move fluid toward the unswollen regions. It can be inferred from the calculations of lymph volume in the subepidermal plexus that the superficial low-pressure massage would be ineffective in decreasing limb circumference. Lymph volume in the dermis is very low and the lymphatic network is interrupted by fibrous elements forming blind lakes. As most tissue fluid accumulates deep in the subcutis and above muscular fascia, high massage pressures should be applied to overcome resistance of fibrotic skin and reach deep limb compartments. Dilated tissue spaces do not contain valves. External pressure applied to the limb surface will also move fluid in a distal direction (Figure 9). This would require special construction of sleeves preventing tissue fluid backflow. How high the massaging pressures should be depends on skin compliance and hydraulic conductivity of the subcutaneous tissue. This is the subject of our current studies.
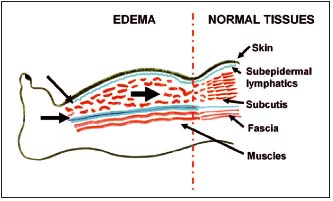
Figure 9. Schematic presentation of the site of accumulation of lymph and free tissue fluid (subepidermal lymphatics, subcutis, and perifascial tissue spaces). Arrows show the direction of flow during pneumatic compression.

REFERENCES
2. Olszewski WL. Episodic dermatolymphangioadenitis (DLA) in patients with lymphedema of the lower extremities before and after administration of benzathine penicillin: a preliminary study. Lymphology. 1996;29(3):126-131.
3. Szczesny G, Olszewski WL. The pathomechanism of posttraumatic edema of the lower limbs: II—Changes in the lymphatic system. J Trauma. 2003;55(2):350-354.
4. Szczesny G, Olszewski WL, Gewartowska M, Zaleska M, Górecki A. The healing of tibial fracture and response of the local lymphatic system. J Trauma. 2007; 63(4):849-854.
5. Lu S, Tran TA, Jones DM, et al. Localized lymphedema (elephantiasis): a case series and review of the literature. J Cutan Pathol. 2009;3:1-20.
6. Füller J, Guderian D, Köhler C, Schneider A, Wendt TG. Lymph edema of the lower extremities after lymphadenectomy and radiotherapy for cervical cancer. Strahlenther Onkol. 2008;184(4):206-211.
7. Pecking AP, Albérini JL, Wartski M, Edeline V, Cluzan RV. Relationship between lymphoscintigraphy and clinical findings in lower limb lymphedema (LO): toward a comprehensive staging. Lymphology. 2008;41(1):1-10.
8. Modi S, Stanton AW, Mortimer PS, Levick JR. Clinical assessment of human lymph flow using removal rate constants of interstitial macromolecules: a critical review of lymphoscintigraphy. Lymphat Res Biol. 2007;5(3):183-202.
9. Bollinger A, Amann-Vesti BR. Fluorescence microlymphography: diagnostic potential in lymphedema and basis for the measurement of lymphatic pressure and flow velocity. Lymphology. 2007;40(2):52-62.
10. Tassenoy A, Vermeiren K, van der Veen P, et al. Demonstration of tissue alterations by ultrasonography, magnetic resonance imaging and spectroscopy, and histology in breast cancer patients without lymphedema after axillary node dissection. Lymphology. 2006;39(3):118-126.
11. Idy-Peretti I, Bittoun J, Alliot FA, Richard SB, Querleux BG, Cluzan RV. Lymphedematous skin and subcutis: in vivo high resolution magnetic resonance imaging evaluation. J Invest Dermatol. 1998;110(5):782-787.
12. Liu N, Wang C, Sun M. Noncontrast three-dimensional magnetic resonance imaging vs lymphoscintigraphy in the evaluation of lymph circulation disorders: A comparative study. J Vasc Surg. 2005;41(1):69-75.
13. Olszewski WL. Atlas of Lymphology, Servier, Paris, 2001.
14. Zerbino DD. Method of investigation of the vessels of the lymphatic vessels. Biull Eksp Biol Med. 1958;45(2):125- 126.
15. Olszewski W, Zajac S, Machowski Z, Sokolowski J. Stainings of the lymphatic vessels by the D. D. Zerbino method. Folia Morphol (Warsz). 1968;27(3):397-402.
16. Fumiere E, Leduc O, Fourcade S, et al. MR imaging, proton MR spectroscopy, ultrasonographic, histologic findings in patients with chronic lymphedema. Lymphology. 2007;40(4):157-162.
