Changes in clinical manifestations and biophysical properties of the great saphenous vein in transient premenstrual phlebopathy after 12 months’ treatment with micronized purified flavonoid fraction
Aleksandr NICOLAICHUK, MD
Omsk State Medical University,
Omsk, Russia
Abstract
Aim. To study clinical manifestations and biophysical properties of the great saphenous vein (GSV) in 42 women with transient premenstrual phlebopathy (TPP) after 12 months of micronized purified flavonoid fraction (MPFF) treatment (1000 mg/day) and to compare GSV diameters measured in 84 legs before 10:00 and after 18:00. In 12 months the number of women with leg heaviness and swelling decreased to 2 (4.8%). The interphase gradient of the circumference (a difference between the circumferences during the secretory and menstrual phases) for the area over the ankle decreased from 6.87 mm to 3.0 mm. During the secretory phase GSV diameter decreased from 6.4 mm to 4.9 mm in the morning and from 7.2 mm to 5.3 mm in the evening. Interphase gradient of GSV diameter (a difference in diameters measured during the secretory and menstrual phases) decreased from 1.2 mm to 0.5 mm in the morning and from 1.1 mm to 0.5 mm in the evening. Only 2 patients out of 18 with an initial evening reflux of 11cm had an evening reflux of not more than 3.5cm. No patients had morning reflux.
Conclusion. Administration of MPFF (1000 mg/day) over 12 months, in the form of intermittent cyclic 15-day courses which started 15 days before menstruation, causes a decrease in leg swelling and elimination of premenstrual leg heaviness in 95.2% of women. It also provides the recovery of GSV diameter along the entire length and a total elimination of premenstrual morning reflux in the secretory phase.
Introduction
Nowadays, functional venous disorders are widespread.1-3 Transient premenstrual phlebopathy (TPP) is considered to be a particular venous problem in fertile women. Its symptoms include leg heaviness, aching pain, leg swelling, and increased tiredness before menstruation that disappear at the beginning of the menstrual cycle. The reason for such a phenomenon is changes in biophysical properties of the venous wall. In TPP, as well as in transient orthodependent evening phlebopathy, venous tone decreases due to the increased creeping ability of the venous wall. To understand the processes that occur in the venous wall during prolonged orthostatic stress, it is useful to borrow the term “creep” from solid-state physics. In solid-state physics the creep of substances, or after effect, or slowly occurring deformation of a solid body, occurs under the influence of a constant load or stress over time. In settings of prolonged vertical load, it is this creep that can lead to a substantial dilation of the vein lumen. However, an additional clinically significant hormone-induced increase in the expansibility of the venous wall during the secretory phase of the menstrual cycle is thought to be a specific feature of TPP.4
In some patients phase venous dilation may be to such an extent that it causes transient premenstrual great saphenous vein (GSV) reflux due to the relative insufficiency of the valves. By contrast with reflux in orthodependent evening phlebopathy, transient premenstrual GSV reflux is registered only during the secretory phase in the morning with minimal orthostatic loading.4
TPP treatment can be motivated by a monthly decrease in physical activity and quality of life.5 Optimistic results of MPFF treatment in premenstrual syndrome5 and a positive experience with MPFF administration in transient orthodependent evening phlebopathy6 created a basis for studying clinical and biophysical peculiarities of MPFF treatment effect in TPP. Such a therapy should take into the consideration the fact that a provoking factor – monthly changes in the endocrine profile which are natural in fertile women – cannot be excluded.
Intermittent cyclic 15-day courses of drug administration prescribed only for the second part of the menstrual cycle are considered to be a peculiarity of the proposed treatment protocol.
Aim: to study changes in clinical manifestations and biophysical properties of the great GSV in TPP of the lower-limbs with a 12-month MPFF treatment in a form of intermittent cyclic 15-day courses.
Material and methods
From 2016 to 2019 a total of 42 women aged from 21 to 40 years (mean age 31.3 ± 8.9) were examined. At the beginning of the study all of them had leg heaviness and swelling which occurred before menstruation and disappeared in the first part of the menstrual cycle.
Inclusion criteria: parous women at their fertile age with a regular menstrual cycle during the last 6 months suffering from leg heaviness and swelling that occur before menstruation and disappears at the beginning of the menstrual cycle with usual daily activity; voluntary informed consent to participate in the study.
Exclusion criteria: regular leg heaviness and swelling which are not associated with the menstrual cycle; chronic venous disease (CVD) C2-C6 (according to the CEAP classification); history of venous thrombosis; lymphedema and lipedema; gynecological disorders; administration of combined oral contraceptive pills (COC); thrombophilia; chronic obstructive pulmonary disease (COPD); extra physical loading.
All women had had 1 to 3 uncomplicated natural births (1.72;95%CI:1.38-1.99). At the beginning of the study their mean body mass index was 25.15 ± 6.13kg/m2. Their mean height was 164.5 ± 4.42. All patients lived in the city, had an office job, and did not go to fitness classes. All women had daily activity and a traditional night’s rest.
During their menstrual cycle all women underwent a clinical and instrumental examination twice: at days 1 to 4 (menstrual phase), and 25 to 28 (secretory phase).7 The intensity of leg heaviness was assessed according to VAS- 10.
The circumference of the area over the ankle and of the muscular part of the calf (its upper third) was measured with a measuring tape. The measuring levels were marked on the skin with indelible ink. The measuring levels were also photographed. The previous study showed that in case of no lymphedema and inflammation the changing increase of the calf volume (first of all its muscular part) within 24 hours is mostly caused by regional venous hypervolemia8
To evaluate the influence of estrogens and progesterone during the secretory phase of the menstrual cycle on changings of the limb circumference, the interphase gradient of the circumference (IGC) was calculated; this is a difference between the circumferences during the secretory and menstrual phases.4
Duplex ultrasound scanning (DUS) of the veins was performed according to the international protocol.9 After a traditional examination, morning and evening DUS results (obtained before 10:00 and after 18:00 during the menstrual (1 to 4 days) and secretory (25 to 28 days) phases were obtained, to study the reaction of the GSV to a prolonged orthostatic loading.7
According to the previous study, in TTP all trunk veins cyclically dilate during the secretory phase; however, this happens to a greater extent with the GSV.4 Therefore, the present research is aimed at monitoring GSV. Its diameter was measured at 1 cm from the saphenofemoral junction (including the GSV reflux zone if any). To have an identical projection of the repeated scanning (in the morning and in the evening during the menstrual and secretory phases before during and after the treatment) in the case of complex leg geometry, the sensor was put in the area with a minimal distance from the skin to the vein in the proximal part of the reflux. This area was marked on the skin with indelible ink and then photographed as well.
The diameters of veins were measured by the same physician with the patient in the upright position with normal breathing, at room temperature.
To identify the pathophysiological GSV features, two calculated values were used. The first constituted the difference in vein diameters measured during the secretory and menstrual phases (interphase gradient of the diameter– IGD). This value allowed assessment of hormonal effects on the vein. The second was the difference in vein diameters measured in the morning and in the evening during the secretory and menstrual phases (orthostatic gradient of “evening-morning” diameter–OGD “evening-morning”). This value aided understanding of the GSV reaction to long orthostatic loading.10
These values integrally characterize the biophysical properties of the vein. The first evaluates the change in the expansibility under the hormonal influence during the secretory phase. The second demonstrates the degree of the creeping ability of the vein, being the value of its gradual expansion at long vertical loading.4
GSV reflux was defined as retrograde flow of >0.5 sec. duration9,11 after a Valsalva maneuver and manual compression and decompression of the distal limb.
Considering the fact that the lesion at phlebopathy is usually of a bilateral nature, the study included GSVs in 84 legs.
All patients had a 12-month MPFF monotherapy in the form of intermittent cyclic 15-day courses which started 15 days before menstruation. The dose of the drug was 1000 mg once a day.
The effectiveness of this protocol was assessed by comparing the results of the examination performed before and after the 3rd and the 12th month of treatment.
The assessment of quality of life during the secretory phase was done according to the CIVIQ-2 basing on pain and physical, social, and psychological factors.12
The safety and tolerability of the drug was studied with active identification of possible digestive complaints, and allergic and other manifestations, as well as general tolerability and significant laboratory safety tests (hematology, medical biochemistry, urinalysis).
The statistical analysis was performed using the nonparametric Wilcoxon test. The value P<0.5 was considered statistically significant. The mean values were determined with the 95%CI.
Results
In 3 months the number of women with leg heaviness during the secretory phase decreased from 42(100%) to 4(9.5%). The intensity of leg heaviness in those women who still had it also decreased from 5.2(95%DI:4.7-5.7) to 0.3 (95%DI:0.0-0.6) (P<0.0001) according to VAS-10 scale. In 12 months the number of women with such a complaint decreased to 2(4.8%), and the intensity in those who still felt heavy legs decreased to 0.1(95%DI:0.0-0.2) (P<0.0001) according to VAS-10 scale.
In 3 months IGC for the area over the ankle decreased (P=0.000004) from 6.87 mm (95%DI,6.31-7.35) to 3.3mm (95%DI,2.9-3.6); for the muscular part of the calf it also reduced (P=0.000004) from 9.7 mm (95%DI,9.1- 10.2) to 2.6 mm (95%DI,2.2-3.0). In 12 months IGC for the area over the ankle decreased (P=0.000004) to 3.0 mm(95%DI,2.8-3.3); for the muscular part of the calf it also reduced (P=0.000004) to 2.4 mm (95%DI,2.1-3.0) (Figure 1).
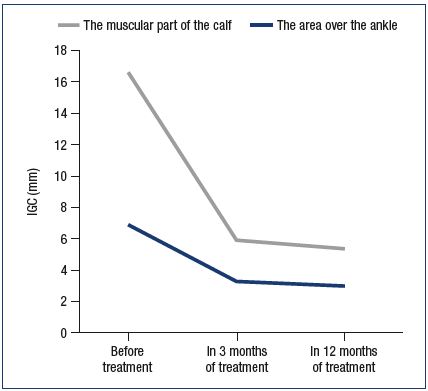
Figure 1. Dynamics of the interphase gradient of the
circumference of the limbs during treatment.
IGC – the interphase gradient of the circumference (cm)
Clinical manifestations of phlebopathy during the secretory phase completely disappeared; moreover, one also noted the decrease in the diameter of GSV(n=84) (Table I). In 3 months of treatment GSV diameter in the groin during the secretory phase reduced from 6.4 mm to 5.0 mm in the morning and from 7.2 mm to 5.4 mm in the evening; OGD also reduced from 0.85 mm to 0.4 mm. A positive effect of MPFF treatment on the expansibility of the venous wall was registered as well: in the morning the IGD of GSV in the groin decreased from 1.2 mm to 0.2 mm, and in the evening from 1.1 mm to 0.2 mm.
In 12 months a further decrease of GSV diameter and the stabilization of biophysical values were noted (Table I, Figures 2 and 3). During the secretory phase GSV diameter in the groin decreased to 4.9 mm in the morning and to 5.3 mm in the evening; OGD value constituted 0.4 mm. A positive effect of MPFF treatment on the expansibility of the venous wall was registered as well: in the morning IGD of GSV in the groin decreased to 0.5 mm, and in the evening–to 0.5 mm.
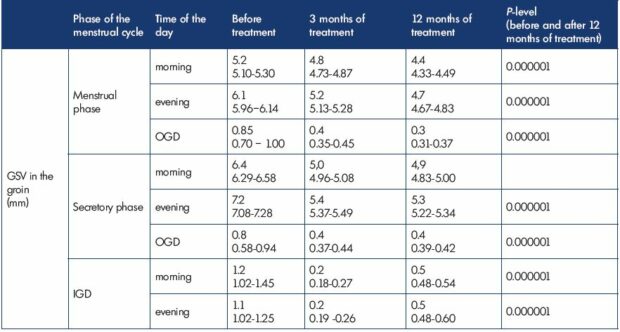
Table I. Great saphenous vein in the groin in transient premenstrual phlebopathy during treatment with micronized purified
flavonoid fraction in the form of intermittent cyclic 15-day courses in the second part of the menstrual cycle (n=84).
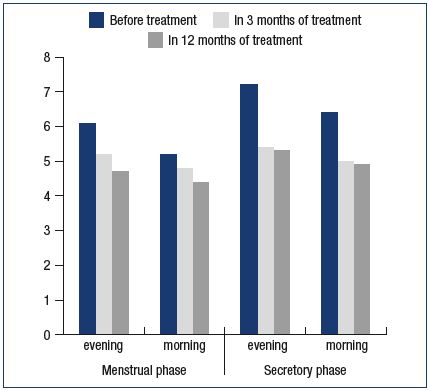
Figure 2. Changes in great saphenous vein (GSV) diameter
in the groin during the menstrual and secretory phases with
micronized purified flavonoid fraction treatment.
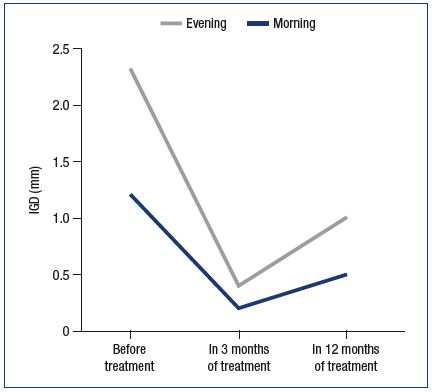
Figure 3. Dynamics of the interphase gradient of great
saphenous vein (GSV) diameter in the groin during treatment.
IGD – the interphase gradient of the diameter of GSV (the
difference in vein diameters measured during the secretory
and menstrual phases)
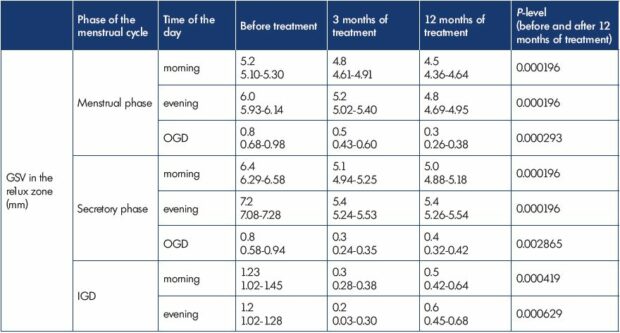
Table II. Great saphenous vein in the reflux zone in transient menstrual phlebopathy during treatment with micronized purified
flavonoid fraction in the form of intermittent cyclic 15-day courses in the second part of the menstrual cycle (n=18).
The GSV reflux zone also showed significant results (n=18) (Table II). In 3 months of treatment GSV diameter during the secretory phase reduced from 6.4 mm to 5.1 mm in the morning and from 7.2 mm to 5.4 mm in the evening. A significant reduction of IGD in the reflux zone was registered as well: from 1.2 mm to 0.3 mm in the morning and from 1.2 mm to 0.2 mm in the evening. In 12 months GSV diameter reduced to 5.0 mm in the morning and to 5.4 mm in the evening; IGD in the morning and in the evening constituted 0.5 mm and 0.6 mm.
Initially during the menstrual phase GSV reflux was absent in all women. During the secretory phase 18 women (42.9%) had an evening reflux of 11.8 cm (95%DI:9.9 -13.7 cm). Of these, 17 patients had a morning reflux of 9.9 cm (95%DI: 5.9-7,8 cm) as well. In all cases it was unilateral and had a segmental nature,13 located in the upper and middle thirds of the thigh.
In 3 months of MPFF treatment no patients had GSV reflux in the morning during the secretory phase; moreover, the number of women with an evening reflux decreased to 6 (14.3). In 12 months of treatment only 2 patients had an evening reflux of not more than 3.5 cm (95%DI:3-6 cm).
Figure 4 shows the initial level of patients’ quality of life (QL) and their levels at 3 and 12 months of treatment. The levels were measured with CIVIQ-2.12 The total score decreased initially from 43.4 (95%DI:41.0-45.8) to 16.5 (95%DI:14.3- 18.6) and further to 13.5 (95%DI:12.1-14,8) (P=0.000001).
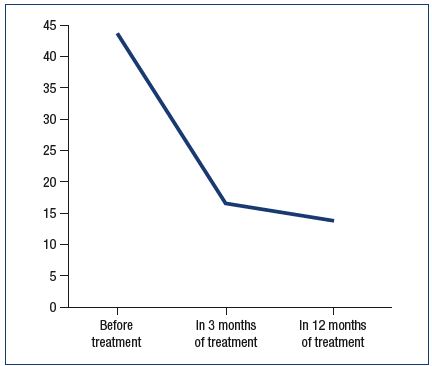
Figure 4. Dynamics of total scores during the secretory phase
according to CIVIQ-2 in patients with transient premenstrual
phlebopathy during treatment.
Any effects that can be considered as side effects were noted only in one patient (2.38%). She complained of a mild epigastric burning and gastralgias during the first week of the drug administration. They disappeared spontaneously, did not renew, and did not require drug withholding. In 12 months any significant changings in cardiac rate and blood pressure, as well as any side effects connected with MPFF administration were not registered. Drug tolerability was of a high level. In 12 months of treatment there were no allergic reactions to the drug. Patient compliance during the whole study was 100%.
Discussion
Earlier we discovered that the decrease in the venous tone at TTP is provoked by changes in two biophysical properties of the venous wall. In TPP, as well as at transient orthodependent evening phlebopathy, creeping ability of the venous wall increases in cases of long orthostatic loading. However, an additional clinically significant hormone-induced increase in the expansibility of the veins during the secretory phase is supposed to be a specific feature of TPP. Such a phase venous dilation is accompanied and followed by premenstrual complaints and symptoms.4 This fact gives an explanation of why the treatment should be provided only during the secretory phase.
Positive results of MPFF administration in the form of intermittent cyclic courses during the second part of the menstrual cycle proved the correctness of theoretical grounds. In 12 months MPFF treatment eliminated premenstrual leg heaviness in 95.2% of women and significantly decreased premenstrual swelling in 100% of cases.
Values before and after treatment showed significant changes in GSV diameter and proved GSV tone recovery during both secretory and menstrual phases. The dynamics of IGD of GSV clearly demonstrated a possibility to stop the development of hormone-induced venous expansibility with a help of medication. At the same time, venous tone recovery occurred to an extent where transient premenstrual GSV reflux was totally eliminated, and GSV diameter in this zone was equal to that in the groin. As a result, patients’ quality of life during the secretory phase improved significantly.
The proposed protocol of treatment based on the study of changes in biophysical properties of veins during the menstrual cycle is completely suitable for the described pathology which occurs due to a long fertility period in women.
It is of note that a decrease in both total dose of the drug and the duration of its continuous administration helped to solve the stated medical problem. This also minimized the risk of side effects, with compliance of 100%.
With a broader understanding of the pathology, which consists of systemic lesions of the venous wall, the results of a 12-month treatment with MPFF were not surprising. With MPFF treatment, venous tone was increased due to the modulation of noradrenergic signaling through the reduced norepinephrine metabolism.14,15
The treatment method proposed in the study differs from a traditional prescription of the drug due to its intermittent cyclic courses connected with the period of maximum progesterone aggression. Such a theoretical background and the chosen treatment guidelines seem to be correct, given that the examined women experienced both subjective and objective improvements.
Administration of MPFF (1000 mg/day) over 12 months in the form of intermittent cyclic 15-day courses in the second part of the menstrual cycle was proven to be safe and well tolerated.
Conclusion
Detailed analysis of biophysical processes in veins at TPP during the menstrual cycle allowed a change in the prescription methods for phlebotropic drugs.
Administration of MPFF (1000 mg/day) over 12 months in the form of intermittent cyclic 15-day courses starting 15 days before menstruation brings about a significant decrease of leg swelling and elimination of premenstrual leg heaviness in 95.2% of women suffering from TPP, and improves patient’s quality of life.
The proposed treatment over 12 months also provides the recovery of GSV diameter along its entire length in menstrual and secretory phases, and a total elimination of a transient premenstrual morning reflux in secretory phase due to a reduction of hormone-induced expansibility of veins.
REFERENCES
1. Bassi G. La patologia venosa funzionale. In: Bassi G, ed. Compendio di terapia flebologica. Turin, Italy: Minerva Medica Ed; 1985.
2. Rabe E, Guex JJ, Puskas A, et al. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31:105-115.
3. Andreozzi GM, Signorelli S, Di Pino L, et al. Varicose symptoms without varicose veins: the hypotonic phlebopathy, epidemiology and pathophysiology. Minerva Cardiangiol. 2000;48:277-285.
4. Tsukanov YT, Nicolaichuk A, Nicolaichuk T. Changes in trunk veins of the lowerlimbs in women with premenstrual leg heaviness and swelling. Int Angiol. 2019;38(2):102-107. doi: 10.23736/ S0392-9590.19.04084-7. [Epub ahead of print]
5. Serfaty D, Magneron AC. Premenstrual syndrome in France: epidemiology and therapeutic effectiveness of 1000 mg of micronized purified flavonoid fraction in 1473 gynecological patients. Contracept Fertil Sex. 1997;25(1):85-90.
6. Tsoukanov YT, Tsoukanov AY, Nikolaychuk A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology. 2015;22(1):18-24.
7. Asbeutah AM, Al-Enezi M, Al-Sharifi NM, et al. Changes in the diameter and valve closure time of leg veins across the menstrual cycle. J Ultrasound Med. 2014;33(5):803-809.
8. Tsoukanov YT. Local venous hypervolemia as a clinical pathophysiological phenomenon of varicose veins. Angiol Sosud Khir. 2001;7:53-57.
9. Coleridge-Smith P, Labropoulos N, Partsch H, Myers K, Nicolaides A, Cavezzi A. duplex ultrasound investigation of the veins in chronic venous disease of the lover limbs-UIP Consensus Document. Part 1. Basic Principles. Eur J Vasc Endovasc Surg. 2006 ;31(1):83-89.
10. Tsukanov YT, Nikolaichuk AI. Orthostaticloading- induced transient venous refluxes (day orthostatic loading test), and remedial effect of micronized purified flavonoid fraction in patients with telangiectasia and reticular vein. Int Angiol. 2017;36(2):189-196. Doi: 10.23736/S0392-9590.16.03708-1
11. Management of chronic venous disease. clinical practice guidelines of European society for vascular surgery. Eur J Vasc Endovasc Surg. 2015;49(6):678-737.
12. Launois R, Reboul-Marty J, Henry B. Construction and validation of a quality of life questionnaire in chronic lowerlimb venous insufficiency (CIVIQ). Qual LifeRes. 1996;5(6):539-554.
13. Engelhorn CA, Manetti R, Baviera MM, et al. Progression of reflux patterns in saphenous veins of women with chronic venous valvular insufficiency. Phlebology. 2012;27(1):25-32. DOI: 10.1258/ phleb.2011.010077.
14. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology. 1997;48(1):45-49.
15. Gargouil YM, Perdrix L, Chapelain B, Gaborieau R. Effects of MPFF at a dose of 500 mg on bovine vessels contractility. Int Angiol. 1989; 8(4 suppl):19-22.
