How to prevent complications and side effects from sclerotherapy of the lower limb veins
Hospital Central de la Cruz Roja, Madrid,
Spain
Abstract
Sclerotherapy is an effective and safe treatment when used by trained and careful hands. Bad results are usually the consequence of an inappropriate use or indication. The best treatment is prevention; however good technique, satisfactory imaging, general precautions, and compliance with posttreatment instructions may prevent some adverse events. Sclerotherapy must be practiced according to the rules of good practice, which is governed by guidelines and international recommendations.
Introduction
If performed properly, sclerotherapy is an efficient treatment method with a low incidence of complications,1-5 but some complications can be vital emergencies. The European guidelines for sclerotherapy in chronic venous disorders recommend considering certain adverse events after sclerotherapy (Table I).4-8 While foam sclerosants does not cause new or different complications vs liquid sclerotherapy, it changes their relative incidence.4 Most adverse effects are minor and inconsequential, such as local injection site pain, urticaria, itching, erythema, and bruising. Other common, but usually self-limiting, side effects include cutaneous hyperpigmentation and telangiectatic matting, or blisters or folliculitis caused by compression postsclerotherapy. Significant and relatively rare complications include systemic life-threatening reactions, anaphylaxis, thromboembolism, cerebrovascular events, tissue necrosis, edema of the injected extremity, and nerve damage.4-8
Adverse effects may be due to the pharmacological properties of the sclerosants, the gas used to produce the foam, the technique of foam production, the injection technique, or postsclerotherapy treatments. Concurrent medical problems, intake of drugs or supplements, and lack of compliance with recommendations are other contributing factors that may also significantly influence the onset of complications.6,9
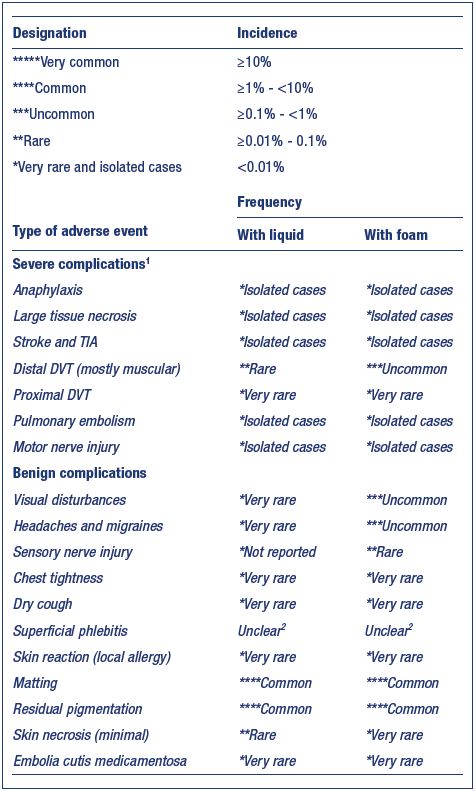
Table I. Complications observed in a prospective French registry
of 12 173 sclerotherapy sessions.
Abbreviations: DVT, deep vein thrombosis.
From reference 4: Guex JJ et al. Dermatol Surg. 2005;31(2):123-
128. © 2005, Blackwell Publishing Ltd.
In order to prevent complications, the European guidelines provide several absolute and relative contraindications to sclerotherapy (grade 1C). Absolute contraindications include known allergies to the sclerosants, acute deep vein thrombosis or a pulmonary embolism, local infection in the area of sclerotherapy or a severe generalized infection, long-lasting immobility and confinement to bed, and, for foam sclerotherapy, the presence of a right-to-left shunt (eg, symptoms of a patent foramen ovale). Relative contraindications (individual benefit-risk assessment is mandatory) include pregnancy, breast feeding, severe peripheral arterial occlusive disease, poor general health, strong predisposition to allergies, high thromboembolic risk (eg, history of thromboembolic events, known severe thrombophilia, hypercoagulation state, and active cancer), acute superficial thrombosis, and, for foam sclerotherapy, neurological disturbances, including migraines, following a previous foam sclerotherapy procedure.
This article reviews the methods for preventing sclerotherapy complications.
Major complications
Systemic allergic reactions caused by sclerotherapy treatment occur very rarely. Local or generalized skin reactions, such as urticaria, are much more frequent (around 0.6%) than systemic involvement, and true anaphylaxis is an extremely rare complication constituting an emergency.9-13 These reactions are unpredictable. Currently, no available methods can identify the individuals who are predisposed to these reactions, meaning that such adverse reactions cannot be prevented. Patients who have undergone multiple previous treatments with liquid sclerosants, those developing postsclerotherapy generalized urticaria, patients with mastocytosis, chronic urticaria, or other urticarial conditions may be at a higher risk.6 Since the risk increases with repeated exposures to the antigen, it is best to always be prepared for these reactions.9 Foam sclerosants are associated with a lower incidence of hypersensitivity reactions compared with liquid sclerosants due to an exposure below the required minimum allergenic dose.10
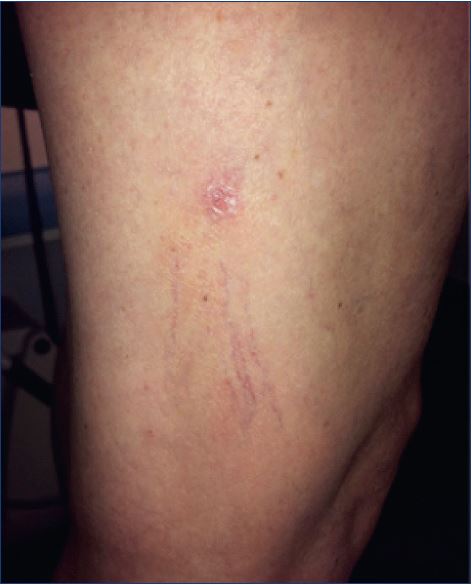
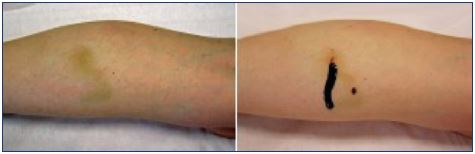
Tissue necrosis most commonly presents as an ulcer, but it can result in extensive loss of tissue (Figure 1). Cutaneous necrosis may occur with the injection of any sclerosant, even under ideal circumstances, and it does not necessarily represent a physician error. Fortunately, its occurrence is rare and usually of limited sequelae.9 Cutaneous necrosis can occur weeks after the initial insult, and is often associated with pain, localized inflammation, and edema. A bright erythema or a prolonged blanching, also described as porcelain-white appearance, may be seen immediately after injection. Pain can be immediate or delayed. Dermal sloughing starts 24 to 72 hours after the ischemic event and the dermis can turn pale or dusky.
Skin necrosis has been described after a paravenous injection of sclerosants in higher concentrations, after injection into a dermal arteriole or an arteriole feeding into a telangiectatic or varicose vein, or after reactive vasospasm of the vessels or venoarterial reflex vasospasm.7,8,14-20 Some classes of sclerosants, such as the chemical irritants and osmotic agents, are more likely to cause tissue necrosis following extravasation.14 The main mechanism leading to tissue necrosis following the use of detergents is arterial occlusion, which may be caused by an inadvertent intraarterial injection or a venoarterial reflex vasospasm.6,15-20 Passage of the sclerosant into the arterial circulation may be mediated by open cutaneous arteriovenous shunts.15-20 Venoarterial reflex vasospasm may result from a high speed or high-pressure injection in small caliber veins, which leads to the rapid dilation of the target vein and vasospasm of the associated arteries. Venoarterial reflex vasospasm clinically presents with prolonged blanching of the skin a few centimeters away from the site of injection, followed by cyanosis and reactive erythema. Prolonged arterial vasospasm may result in tissue infarction and subsequent necrosis.15-20
Prevention
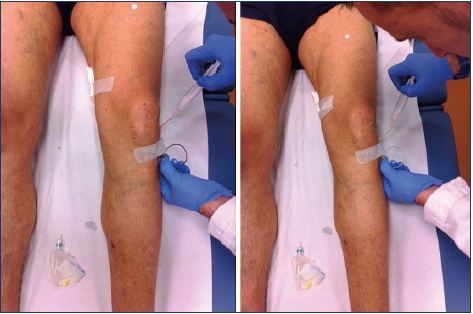
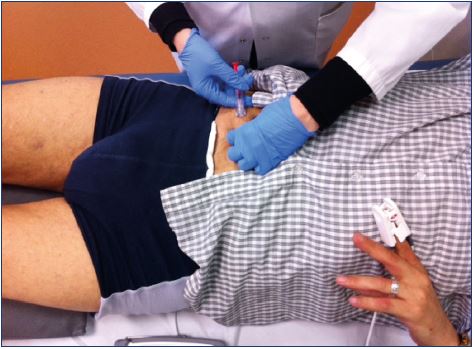
To prevent cutaneous necrosis, a careful and methodical technique must be used: (i) stop injecting if there is a feeling of resistance, if a bleb or wheal forms, or if prolonged blanching occurs; (ii) use the lowest volume and weakest concentration of sclerosant; (iii) avoid using rapid and high pressure injections, especially in telangiectasia and reticular veins, keeping in mind that smaller syringes produce greater pressure; and (iv) use ultrasound-guided sclerotherapy for the deeper reticular veins. These recommendation are classified as grade 1C according to the European guidelines.7,8 An indirect injection and an injection with a transilluminator can help avoid intra-arterial injections or extravasation (Figures 2 and 3).
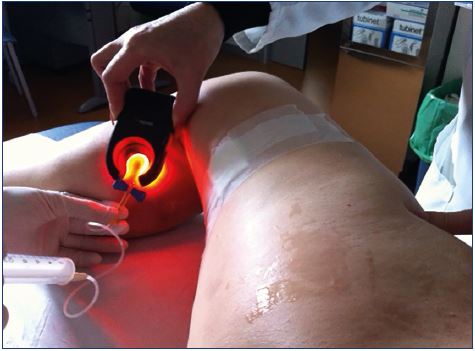
Figure 3. Injection with a transilluminator helps avoid
extravasation and treat the underlying reflux, preventing a
matting appearance.
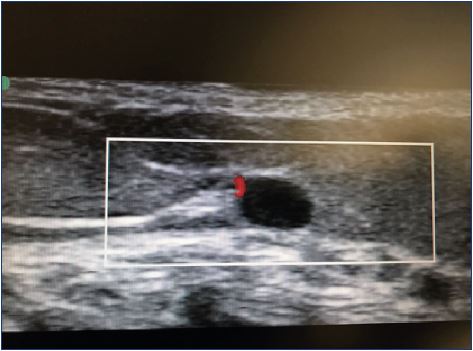
Direct arterial/arteriolar injection is exceptionally rare. In fact, less than 70 cases have been described to date,14,17,23-27 and most of them have occurred after an injection in the ankle region and in the perforating veins above the medial ankle. Other risk areas include the cross-section of the small saphenous vein (Figure 4) and the cross-section of the great saphenous vein. Several cases have involved arterioles of the medial thigh.23-27 Historically, the medial malleolar region was the most common site for intra-arterial injections,19,26 which may relate to direct-vision sclerotherapy in these regions, targeting the posterior tibial artery in a relatively superficial position. Larger arteries, such as the femoral or popliteal artery are fortunately less frequently targeted.27 Likely, target vessels include subcutaneous arterioles, such as those accompanying perforating veins in the medial thigh, the superficial sural artery in the posterior calf, and previously undetected arteriovenous shunts or malformations.14,17 Arterial collaterals masquerading as varicose veins may pose a significant risk.28 Ultrasound guidance has helped minimize the occurrence of this catastrophic event, which most frequently results in limb amputation (52.5%).12,23
The extent of cutaneous necrosis is usually related to the site of injection and the amount of solution injected, and ranges from mild-to-severe necrosis of the skin, subcutaneous tissue, and muscle. This complication can follow direct-vision sclerotherapy, ultrasound-guided sclerotherapy, and even catheter-directed sclerotherapy. Theoretically, visualization of the arteries and veins with duplex-assisted sclerotherapy should negate this risk; however, a number of arterial ulcerations have occurred with this technique. Thus, no technique is completely free from this complication. Sodium tetradecyl sulfate has been more frequently implicated in this complication, accounting for 65% of cases vs 11% with polidocanol.23 High concentrations of sodium tetradecyl sulfate can interact with blood to precipitate an insoluble complex of fibrinogen and Apo lipoprotein B29 and induces platelets to release serotonin, a potent vasoconstrictor, which may contribute to a more prolonged occlusion.25 Other cases reported have involved polidocanol. Both liquid and foam formats have comparable rates of tissue ischemia25,26; hence, practitioners should exercise caution when administering both agents and when using either format.
Intra-arterial injection commonly presents with severe sudden pain at the site of injection that propagates along the artery distribution. Pain can happen quickly or progress over several hours. In rare cases, patients have no complaints of pain and demonstrate only a mild, sharply demarcated erythema that becomes dusky and cyanotic after a few hours.23
Prevention
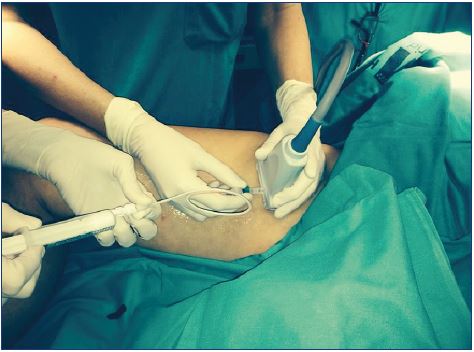
A strong personal experience and a careful ultrasoundguided technique may reduce the risk of this catastrophic complication (Figure 5).23 The European guidelines show that the risk of intra-arterial injection can be minimized using ultrasound guidance with adequate imaging and identification of arteries in close proximity to the target veins. The guidelines recommend using ultrasound guidance for both foam and liquid sclerotherapy when the target vein is not visible or palpable (grade 1C).7,8
The overall frequency of neurological complications of sclerotherapy is around 0% to 2%30,31; the complication include transient events, such as visual disturbances and migraine, as well as ischemic events, such as transient ischemic attacks and stroke, which is an event occurring at a much lower frequency. These complications can result either from a paradoxical clot or from a gas embolism. The most consistent risk factors include a patent foramen ovale or another cardiopulmonary right-to-left shunt.12 The etiology of neurological symptoms following sclerotherapy is currently unknown.
Transient neurologic events: visual disturbances and migraines
A systematic review found that visual disturbances may occur in up to 14% of patients undergoing foam sclerotherapy,32 but a recent systematic review found the overall incidence to be around 1.4%.31 The clinical presentation of these visual disturbances is similar to a classic migraine (with aura).32 Patients presented with a headache alone, isolated visual disturbances in the form of a scotoma or blurred vision, headache combined with visual disturbances, and chest pressure that was either isolated or combined with blurred vision or a scotoma.4,5 Transient neurologic events may be observed after any kind of sclerotherapy, although they occur more frequently after foam sclerotherapy and after treatment of reticular and spider veins.4,5,7,8,32 A session of sclerotherapy for telangiectasia lasts much longer than for a session for varicose veins, and, during this time, the foam can change into liquid with large bubbles.4 All cases spontaneously regressed without aftereffects.
A patent foramen ovale or another right-to-left shunt, which is present in approximately 30% of the general population,33 might be one etiological factor because the pulmonary filter is short-circuited, which allows foam bubbles or endothelin-1 (ET-1) to be released from the injected vessel34 to enter the arterial circulation.30,32,34,36,37 Gillet et al hypothesized that ET-1 reaches the cerebral cortex and induces cortical spreading and triggers a migraine.32 Frullini et al believes that ET-1 provokes a vasospasm, which is the key to understanding migraines, chest tightness, retinal transient ischemia, and neurological ischemia.34,35 There is no clear evidence of a relationship between bubbles and visual or neurological disturbances7,8,36-38; however, bubbles are known to cause vasospasms and may trigger migrainetype symptoms and other general transient effects, such as chest tightness.36 Other factors, such as bubble load, treatment parameters, and patient factors, may also be important.36
Ischemic events: transient ischemic attacks and stroke
The presence of a right-to-left shunt, particularly a patent foramen ovale, is the most consistent risk factor in patients with ischemic neurologic events (transient ischemic attacks and stroke).30 There are only a few published reports of transient ischemic attacks following sclerotherapy,30 and all reported cases were associated with a right-to-left shunt, had an immediate onset, and followed the use of airbased foam sclerosants. It has been suggested that a rightto- left shunt might allow foam bubbles to enter the arterial circulation.30,36-38
Stroke is a very rare, but significant, complication of sclerotherapy.30,39-42,44 Ma et al reported two cases of stroke following 4059 foam procedures in a 6-year period, ie, an incidence rate of 0.01%.41 Parsi reviewed 13 cases of stroke occurring after sclerotherapy published after 199430; 4 cases occurred after liquid sclerotherapy and 9 after foam sclerotherapy, where 3 patients had a partial recovery, while the others had a complete recovery. Cases with an immediate onset following foam sclerotherapy were due to a paradoxical gas embolism,30,39-42,44 while cases with a delayed onset of a few days were due to a paradoxical clot embolism.30,41,42 The mechanism of infarction in a paradoxical gas embolism may be either due to direct physical occlusion of the intracranial arteries by gas bubbles or due to a bubble-induced vasospasm that activates the coagulation system to cause a secondary thrombotic occlusion.30,41 No gas or clot embolism could be demonstrated in 5 of the 13 patients with stroke reviewed (idiopathic and other causes).30,41 The release of cellderived sclerosant byproducts may play a crucial role in the pathogenesis of neurological and other complications of sclerotherapy.30,34,35,45 Finally, a coincidental event due to general causes of stroke should be considered.30
A venous gas embolism presents with dyspnea, continuous cough, hypotension, dizziness, and substernal chest pain. A “mill wheel” murmur may be produced by movement of bubbles in the right ventricle.36 A coronary artery embolism can present with chest tightness and pain, coronary artery spasm, ischemia, arrhythmias, and myocardial infarction. A cerebral gas embolism can present with confusion, focal neurological symptoms, and stroke.30,39-42,44
Prevention
To prevent neurological complications, the following recommendations should be taken into account:
1. Assess the patient for a history of cardiac abnormalities or migraine.
2. Limit the use of foam to large varicose veins, otherwise use a liquid sclerosant for reticular and spider veins.46
3. Prepare the foam using the Tessari method to form the smallest bubbles possible and use the foam within 90 seconds, as bubble coalescence is swift and the stability of sclerosing foam is not great.7,8,46
4. Limit the volume of foam to 10 mL per session.7,8,30,46,47 Technical modifications, such as CO2 or CO2 / O2 foams and Varisolve® polidocanol foam, may allow a larger volume to be injected (Figure 6).46
5. Minimize the bubble load to reduce the passage of the sclerosant into the deep veins through perforating veins and the saphenous junction7,8,30,32 by using multiple low-volume injections48 and injecting only the necessary volume of foam under ultrasound guidance until the target vein is filled.30 Catheterization of the target vein combined with perivenous anesthesia reduces the volume required to achieve vessel closure.49 Sclerotherapy must be administered in a proximal to distal sequence targeting the larger veins and the most proximal sources of reflux first.9,30,36
6. Keep the patient supine for 5 minutes after the injection and avoid having the patient sit or stand up immediately after the procedure (Figure 7) because the risk of a paradoxical gas embolism increases in the sitting position.30,50
7. Avoid manually compressing the saphenous junctions during foam sclerotherapy,30 as this can cause boluses of foam to be released into the central circulation when the pressure is released.51 There have been two published reported cases of a stroke after sclerotherapy.30
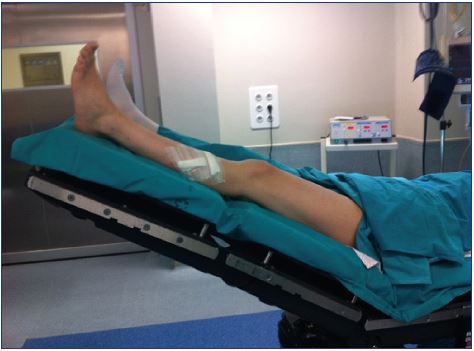
Figure 7. Keep patients supine for 10 minutes after injection and
avoid having the patient sit or stand up immediately after the
procedure.
Figure 8. Compression stockings should be applied by the
medical staff at the end of the procedure to prevent a Valsalva
maneuver.
8. Avoid movements that lead to a Valsalva maneuver during or after the procedures, because this would open a patent foramen ovale or another right-to-left shunt, which is why compression stockings should be applied by the medical staff at the end of the procedure (Figure 8).30
9. A preliminary screening for a patent foramen ovale or a right-to-left shunt is not necessary.7,8
10. Reduce initial volumes and consider using physiologic gases for those patients at an increased risk of neurologic side effects, such as those with a previous classic migraine (with aura) and those with a known patent foramen ovale.46,52-54
11. Patients with a past history of cryptogenic stroke or a history of recurrent classic migraines (with aura) have a higher risk of neurological adverse events and may benefit from preoperative screening and percutaneous closure of a patent foramen ovale.12,30 However, closure procedures are not risk-free,55 and the long-term benefits of a patent foramen ovale closure in sclerotherapy patients is unknown.30
12. Use only liquid sclerosing agents or recommend another kind of treatment for patients with a known symptomatic right-to-left shunt as this is an absolute contraindication for foam sclerotherapy (recommendation grade 1C in the European guidelines).8,46
For patients who have experienced neurological symptoms, including migraines after a previous sclerotherapy session, the European guidelines recommend: (i) the patient should remain lying down for a longer period of time (grade 2C); (ii) avoid injecting large volumes of foam or perform liquid sclerotherapy (grade 2C), although the liquid can also occasionally cause neurologic sequelae in susceptible patients; (iii) the patient should avoid performing a Valsalva maneuver in the early period after the injection (grade 2C); and (iv) on a case-by-case basis, a risk-benefit assessment should be performed based on the particular indication (grade 2C).7,8
Patients with a suspected venous gas embolism should be immediately placed in a left lateral decubitus position to reduce entry into the pulmonary arteries and a possible subsequent right ventricular outflow obstruction.36
Severe deep vein thrombosis, proximal or extensive, is rare. The vast majority of reported deep vein thrombosis cases are localized to the lower legs. The overall frequency of deep vein thrombosis is <1%31,56; however, the incidence is possibly higher as a significant number of procedural deep vein thrombosis may be silent (most reports only include symptomatic cases). The incidence of symptomatic deep vein thrombosis is 0.02% to 0.6%4,31,56 and the incidence with a duplex ultrasonography follow-up is 1.07% to 3.2%.1,4,5,47,57-59 Most of the cases detected by duplex ultrasonography during routine follow-up were asymptomatic.1,4,5,47,57-59 Medial gastrocnemius vein thrombosis is a complication that is more commonly associated with foam sclerotherapy of the small saphenous vein than with the great saphenous vein, likely due to the anatomy of the small saphenous sein.59
Pulmonary embolisms occur very rarely after sclerotherapy. In the study by Gillet,5 only 1 case of pulmonary embolism was reported out of 1025 patients. In a French registry of 12 173 procedures, no cases of pulmonary embolism were reported.4 There is no data regarding the incidence of postoperative silent pulmonary embolisms. Research on the effects of sclerotherapy on coagulation has shown contradictory findings.29,45,59-64 To date, no obvious prothrombotic effect has been demonstrated.9
Prevention
The use of larger volumes of sclerosants, particularly foam, increases the risk of a thrombosis.57,65 Treatments that might influence the risk of deep vein occlusion have been reviewed, and using large total volumes of foam (>10 mL) was identified as a risk factor.57,65 Myers et al showed that varicose veins >5 mm and small saphenous vein treatment were risk factors for deep vein thrombosis.47 He recommends limiting the volume of foam to 1.5 to 2 mL during sclerotherapy of the small saphenous vein.65 The European guidelines recommend avoiding injections near the saphenous junction and perforating veins, if possible, to prevent foam from entering the deep venous system.7,8
To prevent venous thromboembolic complications, the follow recommendations should be taken into account:
1. Use a maximum of 10 mL of foam per session in routine cases (grade 2B). Higher foam volumes can be applied according to an individual risk-benefit assessment (grade 2C)7,8; however, using large total volumes of foam (>10 mL) was identified as a risk factor for deep vein thrombosis.57,65
2. Limit the quantity of sclerosing solution to 0.5 to 1 mL per injection, as multiple small-dose injections using a low volume of foam can reduce the passage of sclerosant foam into the deep veins and decrease venous thromboembolic complications.48,65 In vitro studies by Parsi30 and Watkins66 have shown that the action of sodium tetradecyl sulfate is inhibited by blood proteins and approximately 0.5 to 1 mL of whole blood deactivates 1 mL of 3% sodium tetradecyl sulfate.
3. Implement immediate ambulation after sclerotherapy treatment. Venous stasis is the most likely mechanism for deep vein thrombosis after sclerotherapy. Prolonged immobilization and long-distance travel in the first week after sclerotherapy may increase the risk of a venous thromboembolism (grade 1C).7,8
4. Apply compression using compression stockings or bandages after sclerotherapy (grade 2C).7,8 The critical time for thrombus formation in sclerotherapy treated vessels is approximately 9 hours posttreatment. Some authors suggest that compression stockings or bandages are the most beneficial during the night after sclerotherapy treatment and during other periods of relative vascular stasis when an intravascular thrombus is being formed,9 but there is no evidence to support this idea.
5. Elevate the extremity to 30 degrees and implement immediate ambulation or calf movement with full dorsiflexion of the ankle to empty the deep leg veins, including the muscular and soleal sinuses, as this promotes rapid dilution of the solution from the injected area to decrease the risk of thromboembolic events.9 While this is a common practice in sclerotherapy treatment, it is not supported with any evidence.
6. Use pharmacological thromboprophylaxis in line with current guidelines/recommendations (grade 1C) (Figure 9), implement physical prophylaxis (compression, movement) (grade 1C) (Figure 10), avoid injecting large volumes of foam (grade 1C), decide on a case-by-case basis (benefit-risk assessment) on the particular indication (grade 1C) in patients with risk factors for a venous thromboembolism (eg, high foam volume, overweight, immobility, older age, hormonal treatments, a history or a previous venous thromboembolism event or thrombophilia).7,8,67-70
7. It is not recommended to perform routine investigations for the presence of thrombophilia factors in the coagulation system (Grade 1C).7,8
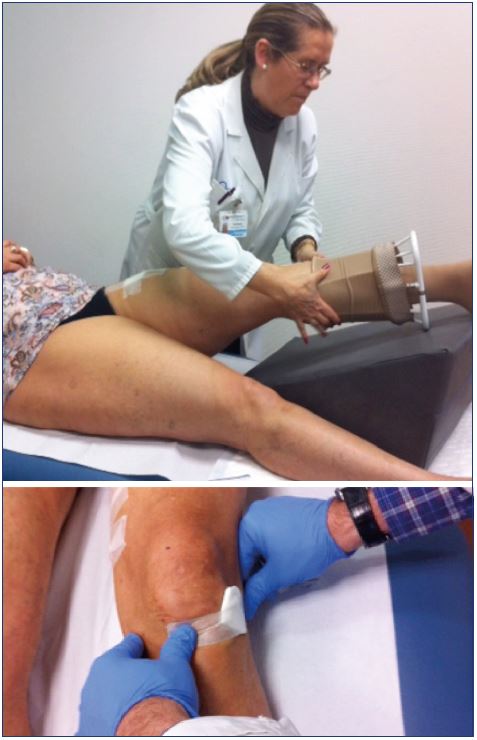
Figure 10 Medical compression systems have anti-inflammatory
effects, decrease chronic venous hypertension, and help resolve
intravascular coagula.
Physical prophylaxis (compression, movement) is recommended
to prevent a venous thromboembolism.
A study by Hamel-Desnos et al67 suggests that, in the three most common forms of thrombophilia (ie, patients with a Factor V Leiden mutation, a prothrombin 20210A mutation, high levels of Factor VIII, or a combination), sclerotherapy, in combination with thromboprophylaxis, can be performed safely.67 The volumes used in this study were low. The authors recommend that the risk-benefit balance should be assessed for each patient. Thrombophilia with a significantly elevated relative risk of a venous thromboembolism or thrombophilia is a contraindication for sclerotherapy. In patients with thrombophilia and an elevated or moderate relative risk who are on long-term oral anticoagulation, sclerotherapy may be given without using low-molecular-weight heparin; however, if these patients are not on oral anticoagulation, then 7 days of low-molecular-weight heparin should be used. In patients with thrombophilia and a moderate relative risk who arenot on oral anticoagulants, low-molecular-weight heparin should be given for 1 to 7 days, depending on the clinical context and medical history.67
Gillet et al showed that, when compared with younger patients, sclerotherapy using low volumes of sclerosant in older patients was not associated with a higher risk of side effects, no specific complications, or need for special precautions.68
The definition of phlebitis after sclerotherapy in the literature is controversial. It is more a part of the treatment process than a complication, and it is considered an adverse event if there is an extension beyond the treated area or an excessive inflammatory reaction.7,8 It is difficult to classify as normal or abnormal when there is either an abnormal extension along the vein or an excessive inflammatory reaction. Venous sclerosis (collagen deposition, which results in scar formation), venous thrombosis (intravascular fibrin clot formation), and venous thrombophlebitis (clot formation accompanied by an inflammatory infiltrate) are histologically separate entities that cannot always be clinically or monographically differentiated. Hence, the incidence depends on individual understandings, meaning that the real frequency is unknown (range, 0% to 45.8%; mean, 4.7%).4,6-8,31 Thrombophlebitis is a complication that should not be taken lightly. If untreated, the inflammation and clot may spread through the perforating veins to the deep venous system. Patients with superficial venous thrombosis have a 5% to 40% chance of developing a deep vein thrombosis.71
Prevention
The cause of thrombophlebitis is related in part to the treatment technique as well as to a lack of adequate postsclerotherapy treatment with adequate compression and frequent ambulation (Figure 10).9 According to Goldman, a decreased incidence of superficial thrombophlebitis may result from a greater degree and length of compression used after sclerotherapy.9 An inadequate degree or length of compression results in excessive intravascular thrombosis. Perivenous inflammation is observed only in the part of the limb not covered by a compression dressing. Thus, to prevent or minimize the development of postsclerosis thrombosis, compression pads and hosiery should be applied over the entire leg and not just over the treated veins.9
However, even when appropriate compression is used, thrombosis and perivascular inflammation may still occur.
Ascending phlebitis in the small saphenous vein or its long tributaries, starting at the upper edge of the compression stocking, is relatively common. Here, the sclerosing action continues up the abnormal vessel (even beyond what apparently is the extent of the abnormality). It is thought that the sclerosing solution destroys damaged endothelium to a greater extent than normal endothelium.9 Therefore, the placement of a foam pad extending above the compression stocking or bandage to create a gradual transition of pressure from a compressed to a noncompressed vein may provide a safety margin, as well as prevent damage to the vein by an abrupt cut-off of the pressure.9
Sclerotherapy using liquid or foam sclerosants is associated with both sensory and motor nerve damage that is usually transient. The incidence is very rare (0.02%) with paresthesia and dysesthesia as the main presenting complaints.72 Due to their close proximity to the veins, the saphenous and sural nerves may be inadvertently injected during sclerotherapy. Injection into a nerve is reportedly very painful and, if continued, may cause anesthesia and sometimes a permanent interruption in nerve function. Occasionally, a patient complains of an area of paresthesia probably caused by perivascular inflammation extending from the sclerosed vein to adjacent superficial sensory nerves.9
Prevention
Nerves are readily visualized on most modern ultrasound systems and inadvertent damage can be mostly avoided (Figure 11).
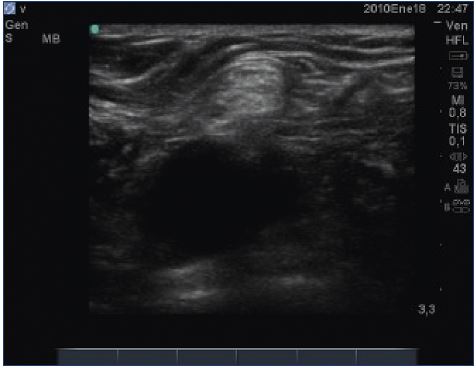
Figure 11. Nerves are readily visualized on most modern
ultrasound systems and inadvertent damage can be mostly
avoided.
The incidence of lower limb edema following sclerotherapy is estimated to be ≈0.5%, but it is rarely reported, meaning that it is probably underestimated.73 This complication is possibly more frequent following obliteration of the small saphenous vein due to the contiguity of this vein with the superficial lymphatic vessels. Localized lymph stasis may occur due to sclerotherapy-induced chemical phlebitis. Extensive sclerotherapy may result in transient lymph stasis in predisposed patients, such as those with latent congenital lymphatic system abnormalities.6 Edema may also be due to deep vein occlusion (thrombosis or sclerosis). Extensive sclerotherapy of superficial incompetent veins followed by occlusion of small segments of the deep veins in the lower limb, such as the posterior tibial or peroneal veins, may also contribute to the edema. In some patients, the etiology is multifactorial and involves a combination of obesity, lack of exercise, concomitant drugs, such as calcium channel blockers, and a lack of compliance with the use of compression stockings.6
Prevention
This complication may be minimized by using careful techniques to avoid phlebitis and deep vein occlusion. Perivascular inflammation must be limited. Ankle edema occurs much less frequently if the sclerosing solution is limited to 1 mL per ankle. Adequate postoperative compression is important in reducing edema and phlebitis in general (Figure 9).6,9,74 One study compared the use of postsclerotherapy graduated compression for a period of 3 days to 3 weeks. In the 10 patients analyzed, 0% reported complaints of edema when the stockings were worn for 3 weeks, 40% of patients who did not wear posttreatment compression stockings had edema, 30% had edema if the stockings were only worn for 3 days, and 20% complained of ankle/pedal edema if the stockings were worn for 1 week.74 Topical application of a potent corticosteroid cream, lotion, or gel is useful.9
Minor complications
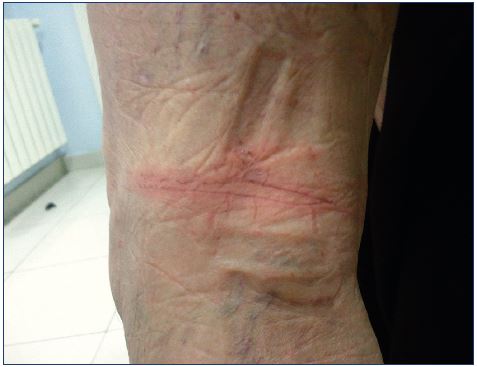
Telangiectatic matting is the proliferation of new vessels (<0.2 mm) in the area of a sclerosed vein; they typically appear 4 to 6 weeks after sclerotherapy treatment (Figure 12),75 and is predominantly seen in women, even though it can also occur in men.75 The most common location is on the inner and outer thighs, near the knees and calves. Unfortunately, even in the most expert of hands, telangiectatic matting may affect one-third of the patients undergoing sclerotherapy, and usually resolves spontaneously in 3 to 12 months.76 In many cases, inadequate or no treatment of the underlying reflux is the cause of telangiectatic matting,77,78 which is especially true when there is underlying saphenous reflux, reticular veins, or when the telangiectasias have been injected directly.77,78
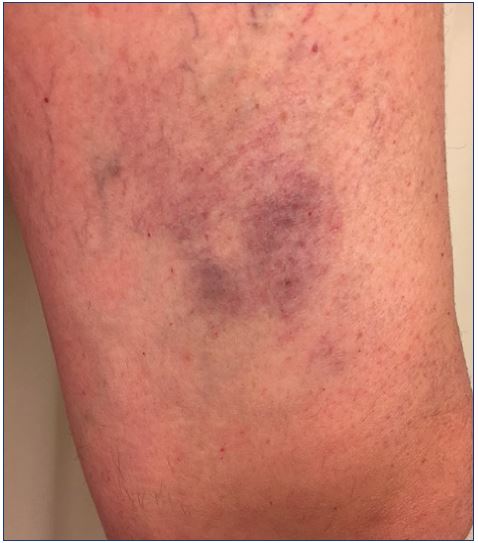
Figure 12. Matting after direct sclerotherapy of reticular veins
and telangiectasias in the presence of an underlying saphenous
reflux.
The precise cause of telangiectatic matting remains unknown; however, its development is attributed to a reactive inflammatory or angiogenic mechanism and is more prevalent with high concentrations or volume of sclerosant or high infusion pressures that can result in inflammation or excessive vein obstruction with subsequent angiogenesis.77,78 Telangiectatic matting is more likely to occur when an increased infusion pressure is used, which results in blanching of the entire capillary network of the skin.75 Patient risk factors include excessive body weight, female sex, hormonal treatments with estrogens, a longer duration of spider veins, and a family history of telangiectasia.75,77,78
Prevention
Efforts to minimize telangiectatic matting are especially important because treatment efforts other than waiting often are not successful. Assess for risk factors prior to treatment. Avoid inadequate treatment of the underlying reflux (Figure 3), use the lowest concentration and volume of the chosen sclerosant that will effectively obliterate the vein, and use the lowest pressure to deliver the sclerosant to minimize excessive vessel injury. When a patient who is taking exogenous estrogen demonstrates a tendency toward telangiectatic matting, consider temporarily stopping the estrogen treatment during the treatment period.75
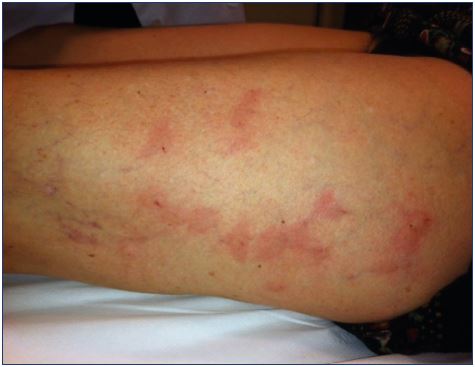
Postsclerotherapy hyperpigmentation refers to the appearance and persistence of pigmentation along the course of a treated vein (Figure 13). Pigmentation occurs in 10% to 30% of patients in the short term, usually appears within 3 to 4 weeks after sclerotherapy, and can last from 6 to 12 months, despite attempts at therapy.9 Although spontaneous resolution occurs in 70% of patients at 6 months, pigmentation may persist longer than 1 year in up to 10% of patients.5,9,77 Hyperpigmentation is usually due to a combination of both melanin and hemosiderin pigment deposits secondary to direct hemosiderin deposition, a postinflammatory processes, or a combination of the two. The red blood cells extravasate after rupture of treated vessels or perivenulitis. The red blood cell dies and the hemoglobin is released into the dermis and is degraded into hemosiderin.8,9
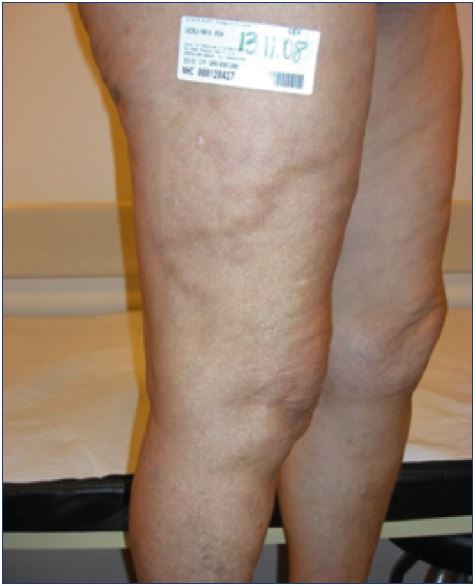
Figure 13. Persisting pigmentation along the course of a treated
vein within 3 to 4 weeks after sclerotherapy.
There is a direct correlation between the incidence of hyperpigmentation and the more concentrated strength or volume of any given sclerosant, however, the incidence varies between the three most commonly used agents.75 A retrospective review showed that there was no difference in the degree or severity of hyperpigmentation between foam and liquid sclerosants.77 A small randomized clinical trial showed there was no difference in hyperpigmentation between sodium tetradecyl sulfate and polidocanol.79 In general, the incidence of hyperpigmentation is higher when treating larger (>1 mm), superficial, and fragile vessels.79
The risk factors that may play a role are a high serum ferritin level, treatment with minocycline, and an intense sun exposure during the treatment process.8,9,80 While there is no general agreement on whether certain skin types are more prone to hyperpigmentation, some authors report a more frequent hyperpigmentation in patients with dark skin and dark hair.4
Prevention
To minimize the risk of developing hyperpigmentation, sclerotherapy should minimize the risk of vessel rupture and red blood cell extravasation and limit endothelial necrosis with its resulting diapedesis of red blood cells by: (i) using a meticulous injection technique2; (ii) avoiding excessive injection pressures by using bigger syringes; (iii) selecting the appropriate solution type, concentration, and dosage in relation to the size and morphology of the vessels to be treated; (iv) using correct therapeutic strategies and tactics, treating areas of venous reflux in a proximal-to-distal manner, and eliminating feeder sources first; (v) aspirating intravascular microthrombi (Figure 14); (vi) prescribing adequate compression therapy (Figure 10).8,9,79
Postsclerotherapy hyperpigmentation tends to be more common with greater amounts of intravascular coagula. Persistent thrombi are thought to produce a subacute perivenulitis that favors extravasation of red blood cells.8 The European guidelines8,80 recommend that intravascular clots should be removed by needle aspiration or stab incision and coagulum expression as soon as possible to reduce the incidence of hyperpigmentation (grade 1C) (Figure 14). Removal of the retained coagulum immediately relieves tenderness and inflammation and may help prevent discoloration.6,80 Microthrombi and larger volumes of intravascular coagulum can be evacuated by puncture with a 16- or 18-gauge needle (depending on the vessel size) and manually expressed or aspired.81 Intervention is recommended within 2 to 4 weeks postsclerotherapy.
Compression stockings minimize the amount of intravascular coagulum, and they have anti-inflammatory effects, decrease chronic venous hypertension, and help resolve the intravascular coagula; therefore, they are an important part of posttreatment care (Figure 10).8 Two randomized clinical trials that compared how compression vs no compression affected the side effects after sclerotherapy (eg, hyperpigmentation, bruising, migraines, and edema) showed no difference in the treatment of telangiectasia, reticular veins,82 or saphenous veins83; however, the evidence is poor. Nonrandomized studies have shown that compression decreases the side effects from sclerotherapy of telangiectasia and reticular veins.74,84,85
Prevention
Preventing the formation of postsclerotherapy-related ecchymosis would theoretically prevent postinflammatory hyperpigmentation by avoiding dermal hemosiderin deposition. Although Arnica Montana is routinely used by many surgeons to prevent perioperative bruising, the efficacy of this homeopathic product has not been scientifically proven.9 Several authors have recommended that patients should avoid taking iron supplements during the course of treatment and for 1 month after treatment.9,86 Izzo et al recommended that patients stop taking drugs that interfere with hemostasis, which eventually lead to bleeding (NSAID, antithrombotic) and drugs and cosmetics that can potentially increase pigmentation (tetracycline, chloroquine, suntan lotion, dyes, bergamot oil).80 European guidelines recommend avoiding UV exposure for the first 2 weeks after sclerotherapy.7,8,80 For high-risk patients, consider another type of treatment or take more rigorous preventive measures.
Intravascular coagulum refers to the common occurrence of palpable intravascular coagulum in a treated vessel, that appears 1 to 6 weeks after sclerotherapy (Figure 14). The intravascular thrombus tends to remain liquefied. In a systematic review of four randomized controlled trials of foam sclerotherapy, the frequency of retained coagulum ranged from 7.8% to 55.1%.31 Intravascular coagulum occurs more frequently in larger blood vessels; coagulum retention is usually associated with tenderness and may predispose the patient to posttreatment pigmentation.
Prevention
Good technique should focus on minimizing the mixture of sclerosant with the intravascular blood, selecting an adequate sclerosant concentration, injecting small volumes from a single point of entry, and applying adequate compression.6 Microthrombi can be minimized with external compression following sclerotherapy (Figure 10).8 It is mandatory to avoid an underlying source of untreated venous insufficiency (Figure 3).81
Transitory general effects are short-lasting disturbances, where recovery occurs within minutes. Chest tightness and dry cough are the most reported effects, but nausea and a metallic taste can also occur. Patients describe two different forms of chest tightness: a simple chest pressure or painful chest tightness. The pathophysiology is not clear. In chest tightness, it is hypothesized that a coronary vasospasm is provoked by air bubbles36 or ET-1 release35; it does not seem to be related to a myocardial infarction and no increase in troponin levels has been observed.63 The type of gas used to prepare foam is a controversial topic. According to Morrison,53 transitory general side effects (tightness, dry cough, and dizziness) are more frequent when injecting a large volume of foam (>15 mL), and the frequency is reduced by substituting CO2 for air. Peterson et al showed no differences in efficacy or side-effects between air and CO2 foam sclerotherapy for reticular veins.54 If high volumes of foam are injected, the use of low nitrogen sclerosing foam reduces the early onset of reversible side effects.53 However, no benefits on transitory general effects in patients treated with either a CO2-O2–based foam or an air-based foam in low volumes have been observed.54
To improve the general safety of foam sclerotherapy the European guidelines recommend: (i) injecting a highly viscous foam into varicose veins (C2) (level 1C); (ii) avoiding patient or leg movement for a few minutes after the injection and avoiding a Valsalva maneuver by the patient (level 1C) (Figure 7-8); and (iii) selecting the best type of gas (air or physiological gas) used to prepare the foam, keeping in mind that this is still a controversial topic (Figure 6).7,8
Stress-related symptoms
Vasovagal reflex is nonspecific and benign, but the patient is at risk for falling. It is the most common cause of a simple loss of consciousness.87 The vasovagal reflex is a common adverse sequelae of any surgical or invasive procedure. It has been estimated to occur in 1% of patients during sclerotherapy9 and must be managed according to the protocol or syncope management.87 A characteristic of a vasovagal response is dysfunction of the autonomic nervous system, with parasympathetic activation that results in initial bradycardia and loss of sympathetic stimulation, which, in turn, causes initial hypotension. An environmental trigger, such as a needle stick, is a common cause.87
Vasovagal reactions typically present with a prodrome of nausea, pallor, and diaphoresis, although a sudden loss of consciousness is also possible. Other common symptoms include lightheadedness, feeling hot, and tinnitus. The patient may also have shortness of breath and palpitations. Lack of blood flow to the brain can result in confusion or even syncope that usually provokes the most concern from the physician and staff.87 As the reaction progresses, a seizure may occur, as well as cardiac arrhythmia with a rapid decrease in cardiac output and even cardiac arrest.9 Vasovagal reactions are most often preceded by a painful injection, but may even occur from the patient seeing the needle or smelling the topical isopropyl alcohol or sclerosing solution.
Prevention
The main concern with a vasovagal reaction is that the patient will fall and sustain injuries. Therefore, both the nurse and physician should watch the patient closely for signs of restlessness, paleness, and excessive perspiration. All patients should be warned to sit down if they become dizzy. It is also helpful for the patient to hold onto an arm rail or other support when needle placement is performed on a standing patient, although treatment while the patient is standing is not recommended. All such reactions are easily reversible when the patient assumes the supine or Trendelenburg position. Preventive measures consist of recommending that the patient eat a light meal before the appointment, maintaining good ventilation in the treatment room, and maintaining constant communication with the patient during the procedure.
Other serious stress-induced problems include exacerbation of certain underlying medical diseases. Wheezing may occur in patients with a history of asthma or angina may develop in patients with cardiovascular disease. Polidocanol is a negative inotropic agent and slows cardiac contractility in a dose-dependent manner.
Urticaria and periorbital edema may be related to histamine release from irritated perivascular mast cells. Urticarial reactions have been rarely observed when using graduated compression stockings. Urticaria may be a sign of a systemic allergy. Therefore, the use of the sclerosing agent in future treatment sessions should be carefully evaluated.
Transitory local side effects
Transient local side effects are common to all sclerosants; they tend to be mild, transient, and somewhat expected. Such complications are usually self-limiting, and they can be treated with topical agents.6 The possible side effects include: (i) injection site reactions (injection pain, minor bruising, urticaria, pruritus, wheals, local swelling, indurations, and erythema) that are self-limited; (ii) skin irritation (itching and an irritant contact dermatitis may follow the use of compression stockings) and excessive xeroderma (dry skin) that can be prevented and/or treated with emollient creams or oils; (iii) tape compression blisters, commonly seen behind the knees, can be prevented by using a tubular support bandage to hold the compression pads instead of tape (Figure 15); (iv) tape compression folliculitis secondary to the occlusion of any hairy area can be prevented by not using tape in hairy areas for a long time; (iv) localized urticarial, often in the form of a wheal associated with itching, is usually relieved within 30 minutes (Figure 16); it can be prevented by applying topical steroids and by limiting the injection quantity per injection site.9
Conclusion
Sclerotherapy is an effective and safe treatment when used by trained and careful hands. Bad results are usually the consequence of an inappropriate use or indication. The best treatment is prevention. Good technique, satisfactory imaging, general precautions, and compliance with posttreatment instructions may help avoid some of the adverse events. Sclerotherapy must be practiced according to the rules of good practice, governed by the respect of the guidelines and international recommendations.
Foam sclerotherapy is a versatile, effective, and generally safe technique used to obliterate incompetent veins. As with every medical treatment, side effects and complications may occur. Fortunately, most of them are benign, but physicians must be aware of the potential serious events and they should be trained to prevent these events. As with general sclerotherapy, bad results from foam sclerotherapy are usually the consequence of an inadequate use or indication. Complications can happen even to the most experienced practitioner. Adequate knowledge of venous anatomy, ultrasonography, and venous hemodynamics and skills with sclerotherapy techniques are paramount. Furthermore, accurate diagnosis, mapping of the reflux pathway, a detailed management strategy that includes an appropriate follow-up; postsclerotherapy treatment should be used to minimize possible complications. Our improved knowledge of complications allows us to implement a careful approach for decreasing their incidence. Treatment techniques should be optimized to reduce the total volume of the sclerosant foam used in each individual treatment session.
According to Hamel-Desnos’ presentation at the 2016 Controversies & Updates In Vascular Surgery meeting, 10 rules must be followed to avoid complications after sclerotherapy:
1. The operator needs to have a good training that is specific to the practice of visual and ultrasound-guided sclerotherapy, a good knowledge of venous disease, and a good practice with venous ultrasound. A regular activity in this practice is paramount.
2. The foam should be made by mixing 1 volume of sclerosing agent with 4 or 5 volumes of gas, with the help of a two-way connector (or a three-way stopcock), it must be of good quality, with no visible bubbles, it must be injected quickly after its preparation to avoid injecting a degraded form, ie, within the shortest possible time between its preparation and its use (<90 seconds). 3. The initial assessment of the pathology must be established in a precise manner to select the best possible tactic, which is adapted to each clinical case. If the incorrect tactic is chosen, then good dexterity is not sufficient. Thus, the choice of the first injection site is decisive, established after a thorough clinical analysis and an ultrasound assessment, and respects the safety of the chosen site. 4. The injections should be administered from the zones of reflux, which are the highest up, toward the distally located veins, and from the largest to the smallest varicose veins. Staged injections allow for the action of the foam, given that the sclerosants is extremely vulnerable once in contact with blood. 5. The choice of the concentration of the sclerosing product is determined according to the diameter of the venous segment to be treated, which is measured while the patient is standing up. 6. The volume injected is determined by the occurrence of a spasm in the target vein and by the homogeneous and compact filling of this vein by the sclerosing foam. The volumes injected are dosed and graduated to avoid overdosing, as opposed to administering a bolus dose at a single point of injection. Treatment techniques should be optimized to reduce the total volume of sclerosant foam used in each individual treatment session. 7. For optimal precision, use a direct needle puncture. 8. Ultrasound guidance should always be used when it is technically possible. It not only provides permanent ultrasound monitoring throughout the procedure, but it is also useful pretreatment (during the assessment and location phases to determine the safety and pertinence of puncture sites) and posttreatment (monitoring of the foam distribution and the occurrence of a spasm in the treated vein). 9. Indications must be correctly targeted, large saphenous veins (>6 mm) can be treated, but this may lead to more recanalizations.
10. The follow-up assessment on the efficiency after foam sclerotherapy should be performed at least 6 weeks after the injection.
REFERENCES
1. Gillet JL. Foam sclerotherapy of saphenous veins—results and side effects. Rev Vasc Med. 2013;1(1):24-29.
2. Rathbun S, Norris A, Stoner J. Efficacy and safety of endovenous foam sclerotherapy: meta-analysis for treatment of venous disorders. Phlebology. 2012;27(3):105-117.
3. Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Endovasc Vasc Surg. 2008;35(2):238- 245.
4. Guex JJ, Allaert FA, Gillet JL, Chleir F. Immediate and midterm complications of sclerotherapy: report of a prospective multicenter registry of 12,173 sclerotherapy sessions. Dermatol Surg. 2005;31(2):123-128.
5. Gillet JL, Guedes JM, Guex JJ, et al. Side-effects and complications of foam sclerotherapy of the great and small saphenous veins: a controlled multicentre prospective study including 1025 patients. Phlebology. 2009;24(3):131- 138.
6. Cavezzi A, Parsi K. Complications of foam sclerotherapy. Phlebology. 2012;27 (suppl 1):46-51.
7. Gillet J; Guideline Group. Complications and side effects: European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(suppl 1):34-38.
8. Rabe E, Breu FX, Cavezzi A, et al; Guideline Group. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338- 354.
9. Goldman MP. Complications and adverse sequelae of sclerotherapy. In: Goldman MP, Weiss R, eds. Sclerotherapy: Treatment of Varicose and Telangiectatic Leg Veins. 6th ed. Elsevier Inc; 2017:200-261.
10. Bunke-Paquette N. Complications of liquid sclerotherapy. In: Bergan JJ, Bunke- Paquette N, eds. The Vein Book. 2nd ed. New York, NY: Oxford University Press; 2014:115-126.
11. Brzoza Z, Kasperska-Zajac A, Rogala E, Rogala B. Anaphylactoid reaction after the use of sodium tetradecyl sulfate: a case report. Angiology. 2007;58(5):644- 646.
12. Parsi K. Emergencies in phlebology: anaphylaxis, intraarterial injection, neurological and cardiac repercussions. Phlebolymphology. 2014;21(1):65-66.
13. Scurr JRH, Fisher RK, Wallace SB, Gilling-Smith GL. Anaphylaxis following foam sclerotherapy: a life threatening complication of a non-invasive treatment for varicose veins. Eur J Vasc Endovasc Surg. 2007;13:87-89.
14. Bergan JJ, Weiss RA, Goldman MP. Extensive tissue necrosis following highconcentration sclerotherapy for varicose veins. Dermatol Surg. 2000;26(6):535- 542.
15. Schuller-Petrović S, Pavlović MD, Neuhold N, Brunner F, Wölkart G. Subcutaneous injection of liquid and foamed polidocanol: extravasation is not responsible for skin necrosis during reticular and spider vein sclerotherapy. J Eur Acad Dermatol Venereol. 2011;25(8):983-986.
16. Miyake RK, King JT, Kikuchi R, Duarte FH, Davidson JR, Oba C. Role of injection pressure, flow and sclerosant viscosity in causing cutaneous ulceration during sclerotherapy. Phlebology. 2012;27(8):383-389.
17. Tran D, Parsi K. Veno-arteriolar reflex vasospasm of small saphenous artery complicating sclerotherapy of the small saphenous vein. Aust NZ J Phlebology. 2007;10(1):29-32.
18. Bihari I, Magyar E. Reasons for ulceration after injection treatment of telangiectasia. Dermatol Surg. 2001;27(2):133-136.
19. Parsi K, Partsch H, Rabe E, Ramelet AA. Reticulate eruptions. Part 1: vascular networks and physiology. Australas J Dermatol. 2011;52(3):159-166.
20. Parsi K, Bartsch H, Rabe E, Ramelet AA. Reticulate eruptions: part 2. Historical perspectives, morphology, terminology and classification. Australas J Dermatol. 2011;52(4):237-244.
21. Zimmet SE. The prevention of cutaneous necrosis following extravasation of hypertonic saline and sodium tetradecyl sulfate. J Dermatol Surg Oncol. 1993;19(7):641-646.
22. Brown AS, Hoelzer DJ, Piercy SA. Skin necrosis from extravasation of intravenous fluids in children. Plast Reconstr Surg. 1979;64(2):145-150.
23. Hafner F, Froehlich H, Gary T, Brodmann M. Intra-arterial injection, a rare but serious complication of sclerotherapy. Phlebology. 2013;28(2):64-73.
24. Fegan WG, Pegum JM. Accidental intraarterial injection during sclerotherapy of varicose veins. Br J Surg. 1974;61(2): 124-126.
25. Parsi K, Hannaford P. Intra-arterial injection of sclerosants: report of three cases treated with systemic steroids. Phlebology. 2016;31(4):241-250.
26. Nitecki SS, Bass A. Inadvertent arterial injury secondary to treatment of venous insufficiency. Vascular. 2007;15(1):49-52.
27. Biegeleisen K, Neilsen RD, O’Shaughnessy A. Inadvertent intraarterial injection complicating ordinary and ultrasound-guided sclerotherapy. J Dermatol Surg Oncol. 1993;19(10): 953-958.
28. Jones L, Parsi K. Arteries masquerading as varicose veins: a trap for phlebologists. Phlebology. 2014;30(10):729-735.
29. Parsi K, Exner T, Low J, Ma DD, Joseph JE. In vitro effects of detergent sclerosants on antithrombotic mechanisms. Eur J Vasc Endovasc Surg. 2009;38(2):220-228.
30. Parsi K. Paradoxical embolism, stroke and sclerotherapy. Phlebology. 2012;27(4):147-167.
31. Jia X, Mowatt G, Burr JM, Cassar K, Cook J, Fraser C. Systematic review of foam sclerotherapy for varicose veins. Br J Surg. 2007;94(8):925-936.
32. Gillet JL, Donnet A, Lausecker M, Guedes JM, Guex JJ, Lehmann P. Pathophysiology of visual disturbances occurring after foam sclerotherapy. Phlebology. 2010;25(5):261-266.
33. Lynch JJ, Schuchard GH, Gross CM, Wann LS. Prevalence of right to left atrial shunting in a healthy population: detection by Valsalva maneuver contrast echocardiography. Am J Cardiol. 1984;53(10):1478-1480.
34. Frullini A, Barsotti MC, Santoni T, Duranti E, Burchielli S, Di Stefano R. Significant endothelin release in patients treated with foam sclerotherapy. Dermatol Surg. 2012;38(5):741-747.
35. Frullini A, Felice F, Burchielli S, Di Stefano R. High production of endothelin after foam sclerotherapy: a new pathogenetic hypothesis for neurological and visual disturbances after sclerotherapy. Phlebology. 2011;26(5):203-208.
36. Parsi K. Venous gas embolism during foam sclerotherapy of saphenous vein despite recommended treatment modifications. Phlebology. 2011;26(4):140-147.
37. Raymond-Martimbeau P. Transient adverse events positively associated with patent foramen ovale after ultrasoundguided foam sclerotherapy. Phlebology. 2009;24(3):114-119.
38. Morrison N, Neuhardt DL. Foam sclerotherapy: cardiac and cerebral monitoring. Phlebology. 2009;24(6): 252-259.
39. Asbjornsen CB, Rogers CD, Russell BL. Middle cerebral air embolism after foam sclerotherapy. Phlebology. 2012;27(8):430-433.
40. Leslie-Mazwi TM, Avery LL, Sims JR. Intra-arterial air thrombogenesis after cerebral air embolism complicating lower extremity sclerotherapy. Neurocrit Care. 2009;11(2):247-250.
41. Ma RW, Pilotelle A, Paraskevas P, Parsi K. Three cases of stroke following peripheral venous interventions. Phlebology. 2011;26(7):280-284.
42. Hanish F, Müller T, Krivokuca M, Winterholler M. Stroke following vatical sclerotherapy. Eur J Med Res. 2004;9(5):282-284.
43. Gillet JL. Three cases of stroke following peripheral venous interventions. Phlebology. 2013;28(1):55.
44. Bush RG, Derrick M, Manjoney D. Major neurological events following foam sclerotherapy. Phlebology. 2008;23(4):189-192.
45. Parsi K, Connor DE, Pilotelle A, Low J, Ma DD, Joseph JE. Low concentration detergent sclerosants induce platelet activation but inhibit aggregation due to suppression of GPIIb/IIIa activation in vitro. Thromb Res. 2012;130(3):472-478.
46. Guex JJ. Complications and side-effects of foam sclerotherapy. Phlebology. 2009;24(6):270-274.
47. Myers KA, Jolley D, Clough A, Kirwan J. Outcome of ultrasound-guided sclerotherapy for varicose veins: mediumterm results assessed by ultrasound surveillance. Eur J Vasc Endovasc Surg. 2007;33(1):116-121.
48. Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Multiple smalldose injections can reduce the passage of sclerosant foam into deep veins during foam sclerotherapy for varicose veins. Eur J Vasc Surg. 2009;37(3):343-348.
49. Parsi K. Catheter-directed sclerotherapy. Phlebology. 2009;24(3):98-107.
50. Morrison N, Neuhardt DL, Rogers C, McEown J, Vollis K. Studies on foam migration. Phlebology. 2009;24:88.
51. Hill D, Hamilton R, Fung T. Assessment of techniques to reduce sclerosant foam migration during ultrasound-guided sclerotherapy of the great saphenous vein. J Vasc Surg. 2008;48(4):934-939.
52. Wong M. Should foam made with physiologic gases be the standard in sclerotherapy? Phlebology. 2015;30(9):580-586.
53. Morrison N, Neuhardt DL, Rogers CR, et al. Comparisons of side effects using air and carbon dioxide foam for endovenous chemical ablation. J Vasc Surg. 2008;47(4):830-836.
54. Peterson JD, Goldman MP. An investigation of side-effects and efficacy of foam-based sclerotherapy with carbon dioxide or room air in the treatment of reticular leg veins: a pilot study. Phlebology. 2012;27(2):73-76.
55. Gryglas A, Smigiel R. Migraine and stroke: what’s the link? What to do? Curr Neurol Neurosci Rep. 2017;17(3):22.
56. Dermody M, Schul MW, O’Donnell TF. Thromboembolic complications of endovenous thermal ablation and foam sclerotherapy in the treatment of great saphenous vein insufficiency. Phlebology. 2015,30(5)357-364.
57. Kulkarni SR, Messenger DE, Slim FJ, et al. The incidence and characterization of deep vein thrombosis following ultrasound-guided foam sclerotherapy in 1000 legs with superficial venous reflux. J Vasc Surg Venous Lymphat Disord. 2013;1(3):231-238.
58. Guex JJ, Schliephake DE, Otto J, Mako S, Allaert FA. The French polidocanol study on long-term side effects: a survey covering 3,357 patient years. Dermatol Surg. 2010;36(suppl 2):993-1003.
59. Gillet JL, Lausecker M, Sica M, Guedes JM, Allaert FA. Is the treatment of the small saphenous veins with foam sclerotherapy at risk of deep vein thrombosis? Phlebology. 2014;29(9):600- 607.
60. Parsi K, Exner T, Connor DE, Herbert A, Ma DD, Joseph JE. The lytic effects of detergent sclerosants on erythrocytes, platelets, endothelial cells and microparticles are attenuated by albumin and other plasma components in vitro. Eur J Vasc Endovasc Surg. 2008;36(2):216-223
61. Hamel-Desnos CM, Desnos PR, Ferre B, Le Querrec A. In vivo biological effects of foam sclerotherapy. Eur J Vasc Endovasc Surg. 2011;42(2):238-245.
62. Connor DE, Joseph JE, Exner T, Ma DDF, Parsi K. Infusion of foam sclerosant results in a distance-dependent procoagulant activity, haemoconcentration and elevation of D-dimer levels. Phlebology. 2014;29(10):677-687.
63. Fabi SG, Peterson JD, Goldman MP, Guiha I. An investigation of coagulation cascade activation and induction of fibrinolysis using foam sclerotherapy of reticular veins. Dermatol Surg. 2012;38(3):367-372.
64. Shadid NH, van der Velden SK, van Oerle R, ten Cate H, Sommer A, Nelemans P. In vivo effects of foam sclerotherapy on coagulation. Phlebology. 2014;29(5):287-292.
65. Myers KA, Jolley D. Factors affecting the risk of deep venous occlusion after ultrasound-guided sclerotherapy for varicose veins. Eur J Vasc Endovasc Surg. 2008;36(5):602-605.
66. Watkins MR. Desactivation of sodium tetradecyl sulphate injection by blood proteins. Eur J Vasc Endovasc Surg. 2011;41(4):521-525.
67. Hamel-Desnos CM, Gillet JL, Desnos PR, Allaert FA. Sclerotherapy of varicose veins in patients with documented thrombophilia: a prospective controlled randomized study of 105 cases. Phlebology. 2009;24(4):176-182.
68. Gillet JL, Desnos CH, Lausecker M, Daniel C, Guex JJ, Allaert FA. Sclerotherapy is a safe method of treatment of chronic venous disorders in older patients: a prospective and comparative study of consecutive patients. Phlebology. 2016;32(4):234-240.
69. Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg. 2006;32(5):577-583.
70. Holbrook A, Schulman S, Witt DM, et al. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e152S-e184S.
71. Galanaud JP, Gentry C, Sevestre MA, Brisot D, et al; OPTIMEV SFMV Investigators. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thrombotic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost. 2011;105(1):31-39.
72. Bergan J, Pascarella L, Mekenas L. Venous disorders: treatment with sclerosant foam. J Cardiovasc Surg. 2006;47(1):9-18.
73. Cavezzi A, Frullini A, Ricci S, Tessari L. Treatment of varicose veins by foam sclerotherapy: two clinical series. Phlebology. 2002;17(1):13-18.
74. Weiss RA, Sadick NS, Goldman MP, Weiss MA. Post-sclerotherapy compression: controlled comparative study of duration of compression and its effects on clinical outcome. Dermatol Surg. 1999;25(2):105-108.
75. Goldman MP, Sadick NS, Weiss RA. Cutaneous necrosis, telangiectatic matting, and hyperpigmentation following sclerotherapy. Etiology, prevention, and treatment. Dermatol Surg. 1995;21(1):19-29.
76. Munavalli GS, Weiss RA. Complications of sclerotherapy. Semin Cutan Med Surg. 2007;26(1):22-28.
77. Palm MD, Guiha IC, Goldman MP. Foam sclerotherapy for reticular veins and nontruncal varicose veins of the legs: a retrospective review of outcomes and adverse effects. Dermatol Surg. 2010;36(suppl 2):1026-1033.
78. Kern P, Ramelet AA, Wutschert R, Bounameaux H, Hayoz D. Singleblind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30(3):367-372.
79. Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatol Surg. 2005;31(6):631-635.
80. Izzo M, Mariani F, Binaghi F, Amitrano M. Postsclerotherapy hyperpigmentation: incidence, clinical features, and therapy. In: Negus D, Jantet G, Coleridge-Smith PD, eds. Phlebology ’95. Springer-Verlag; 1995:550-552
81. Scultetus AH, Villavicencio JL, Kao TC, et al. Microthrombectomy reduces postsclerotherapy pigmentation: multicenter randomized trial. J Vasc Surg. 2003;38(5):896-903.
82. Kern P, Ramelet AA, Wütschert R, Hayoz D. Compression after sclerotherapy for telangiectasias and reticular leg veins: a randomized controlled study. J Vasc Surg. 2007;45(6):1212-1216.
83. Hamel-Desnos CM, Guias BJ, Desnos PR, Mesgard A. Foam sclerotherapy of the saphenous veins: randomised controlled trial with or without compression. Eur J Vasc Endovasc Surg. 2010;39(4):500- 507.
84. Goldman MP, Beaudoing D, Marley W, Lopez L, Butie A. Compression in the treatment of leg telangiectasia: a preliminary report. J Dermatol Surg Oncol. 1990;16(4):322-325.
85. Nootheti PK, Cadag KM, Magpantay A, Goldman MP. Efficacy of graduated compression stockings for an additional 3 weeks after sclerotherapy treatment of reticular and telangiectatic leg veins. Dermatol Surg. 2009;35(1):53-57.
86. Thibault PK, Wlodarczyk J. Correlation of serum ferritin levels and postsclerotherapy pigmentation. A prospective study. J Dermatol Surg Oncol. 1994;20(10):684 -686.
87. Mowatt-Larssen E. Syncope for phlebologists. Phlebology. 2014;29(8): 517-521.