Small saphenous vein interventional treatment
Hospital, Paris XII University, Créteil, France
Abstract
Evidence-based medicine can provide some clues about options for treating varicose veins, but there is no consensus on the best option. Even though the majority of practitioners have discarded several of the newer techniques available after having used them, they can nonetheless remain good options for others. However, the best procedure would be easily reproducible by the majority and for the majority. The adoption of these new and less invasive techniques, such as chemical ablation (foam sclerotherapy) and endovenous thermal ablation, could allow for treatment at a private doctor’s surgery, thereby reducing the risk of recurrence and paresthesia that are often associated with traditional surgery under general or spinal anesthesia. Even though foam sclerotherapy is one of the best options for treating the small saphenous vein, thermal ablation, including endovenous laser ablation, can be more efficient for larger veins, regardless of the vein anatomy. The ideal treatment for varicose veins in all cases should be an ambulatory procedure without anesthesia or only local anesthesia because this will not require the patients to stop work or need nursing care. Due to the reduced convalescence, pain, and morbidity, thermal ablation has been graded 1B according to the 2011 American guidelines, 1B according to the 2014 European Venous Forum, and recommended by the 2013 NICE guidelines as a first-choice procedure.
Introduction
Great saphenous vein incompetence is the most frequent cause of varicose vein disease; however, small saphenous vein incompetence occurs in about 20% of patients presenting with varicose veins.1,2 Treating the small saphenous vein must be carried out very carefully, even more so than for the great saphenous vein, because the ending is variable and it is in close proximity to the nerves.
Conventional surgery, which has been the only treatment for small saphenous vein incompetence, is being challenged due to the high incidence of recurrence and the frequently associated postoperative complications. Due to hospitalization, general or spinal anesthesia, and too many days of sick leave, traditional surgery could be replaced with less invasive methods.
Chemical ablation is widely accepted as a safe and effective treatment for the small saphenous vein and especially for small and medium-sized veins. However, while ultrasound guided foam sclerotherapy is safer than conventional surgery, it may result in rare, but major complications and litigation claims. During liquid or foam sclerotherapy, vascular physicians are afraid of mistakenly injecting the artery companion to the small saphenous vein. Therefore, to prevent this complication, the veins and arteries should be mapped carefully by using duplex ultrasound to locate the exact position of the arteries and avoid the high-risk zones.
Thermal ablation–endovenous laser ablation or radiofrequency ablation–is a minimally invasive technique that is mostly used for the great saphenous vein. However, for the small saphenous vein, ablation with the endovenous laser is preferentially used. We identified 15 specific studies on endovenous laser ablation of the small saphenous vein (Table I).3-17 The guidelines recommend mapping prior to any type of saphenous treatment, which is particularly true for the small saphenous vein. Now, due to improved duplex ultrasound technology, the small saphenous vein’s nerves can be identified and mapped.
Treating the small saphenous vein
using endovenous laser ablation
The procedure is performed entirely under duplex ultrasound guidance. The varicose vein is punctured at the distal insufficiency point with a 19G (or 21G) needle. Then a guide wire is inserted through the needle, and, after removing it, a 6Fr (or a 4Fr) introducer sheath is placed over the guide wire into the vein. The guide wire is then replaced by a 600 μm or 400 μm laser fiber that is positioned accurately at the saphenopopliteal junction. Tumescent anesthesia is administered around and along the small saphenous vein. Laser energy is delivered using a diode laser generator and the small saphenous vein is ablated during withdrawal of the fiber.
I began practicing endovenous laser ablation in June 2001 with a 980 nm and bare-tipped fiber, and, in June 2007, with a 1470 nm and bare-tipped fiber, and then, in June 2008, with a 1470 nm and radial fiber. This treatment is designed for symptomatic patients and a small saphenous vein trunk diameter >5 mm. The endovenous laser ablation procedure must be standardized in terms of the access site, positioning of the fiber tip, tumescent anesthesia (20 cc lidocaine [1% dilute in 250 or 500 cc of cooled saline] without bicarbonate or epinephrine), and energy according to the size of the vein needs to be very precise. Mapping both the vein and nerves prior to the procedure should become a routine practice.
Anesthesia
Before beginning thermal ablation, the entire length of the small saphenous vein to be treated is surrounded by a dilute local anesthetic that is injected at several points in the leg. Tumescent anesthesia is recommended for four reasons: (i) anesthesia reasons; (ii) to protect the surrounding tissues; (iii) to spasm the vein (for the treatment, it is better to have less blood in the vein); and (iv) to keep the patient conscious to stop the procedure when it is painful, thereby avoiding nerve damage. During tumescent anesthesia and under ultrasound guidance, the position of the nerves, previously identified, determines a safe puncture area, which is located a certain distance away from the nerves. The patient is only under local anesthesia, which may be associated with light sedation. General or spinal anesthesia should be avoided because they provoke vasoplegia (dilation of the vein), which reduces the treatment’s efficacy. In addition, the patient is unable to feel anything, which increases the risk of nerve damage.
Access site
The access site is an important part of the procedure that must be considered. While the endovenous procedure is well documented in scientific literature regarding catheterization, tumescent anesthesia, positioning of the fiber tip, and the use of ultrasound guidance, there is little information available on the ideal puncture site. It is current practice to access the main trunk of the great saphenous vein or the small saphenous vein at the lowest incompetence area. In fact, the key point is where to begin the endovenous procedure. Catheterization should occur at the lowest part of the small saphenous vein incompetency, but the access should occur at an incompetent tributary.18 The goal is to disconnect the competent part of the small saphenous vein from the incompetent part (Figure 1). If we catheterize the small saphenous vein at the lowest incompetence area and above an incompetent tributary, then, after the treatment, the blood will flow from the competent small saphenous vein toward the tributary, which will necessitate a phlebectomy of this tributary. If we access the small saphenous vein at the lowest point of incompetence, but away from the incompetent tributary (Figure 2), then we are treating both the small saphenous vein and tributary at the same time, which avoids a phlebectomy. In our practice, the access site is crucial.
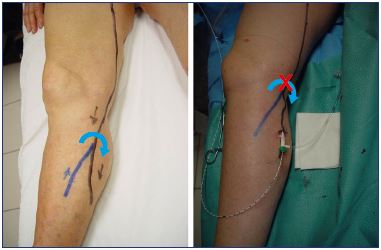
Figure 1. Introduction through the tributary to disconnect the
competent part from the incompetent part.
How to avoid neurologic risks
Fifteen studies on endovenous laser ablation of the small saphenous vein (Table I),3-17 including two randomized clinical trials (endovenous laser ablation vs surgery)16,17 and one meta-analysis (Table II),19 show that the rate of paresthesia is between 1.3% and 11% with only sensory damage (no motor nerve lesions). This rate was low, 4% on average, except for one study18 in which the rate was up to 40%, but only for 2 weeks. Even if the paresthesia rate remains very low, irrespective of the nerves, it could be even lower if the small saphenous vein and the nerves are mapped prior to the procedure.
The sciatic nerve, which is located posterior to the thigh, is the largest nerve with a diameter >1 cm. It is divided at variable levels, but mostly at the summit of the popliteal fossa (a minimum of 3.5 cm above the popliteal skin crease). This division is slightly displaced (around 1.5 cm) from the longitudinal axis of the limb on the lateral aspect. It is divided into 2 nerves: the tibial nerve and the fibular nerve (Figure 3).
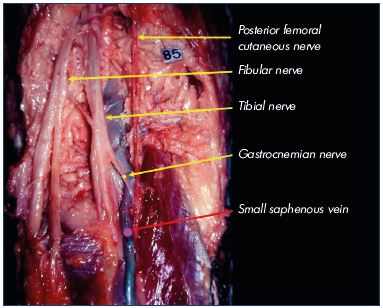
Figure 3. Division of the sciatic nerve slightly displaced from the
longitudinal axis of the limb (the posterior femoral cutaneous
nerve gives the midline of the limb).
Image courtesy of Prof Gillot.
The medial sural nerve, which arises from the tibial nerve, lies deep under the deep fascia on the middle of the popliteal fossa and then goes slightly toward the medial aspect of the middle third of the calf. At an indeterminate location, mostly below the groove of the two heads of the gastrocnemius muscle, the medial sural nerve pierces the deep fascia and joins the small saphenous vein in the superficial tissues. The lateral sural nerve, which arises from the fibular nerve above the popliteal crease, lies on the superficial fascia on the lateral aspect of the leg, then goes medially to join the small saphenous vein at an indeterminate location, sometimes between two of the aponeurosis layers of the small saphenous vein. It joins the medial sural nerve mostly at the lower third of the calf to form one nerve, known as the sural nerve, which could be wrapped around the small saphenous vein.
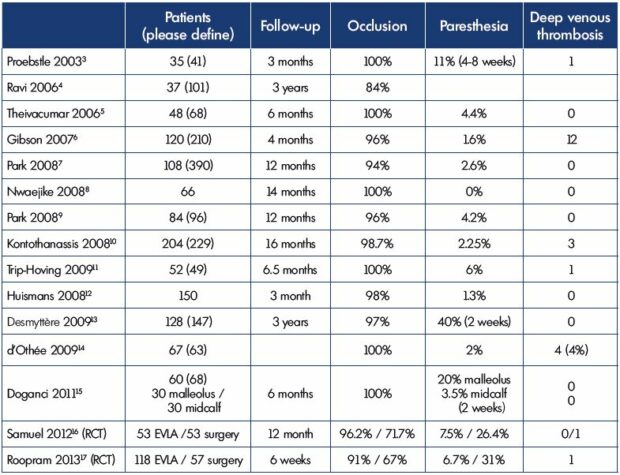
Table I. Specific studies on EVLA of the small saphenous vein: follow-up, occlusion rates, and paresthesia.
Abbreviations: EVLA, endovenous laser ablation; RCT, randomized control trial.
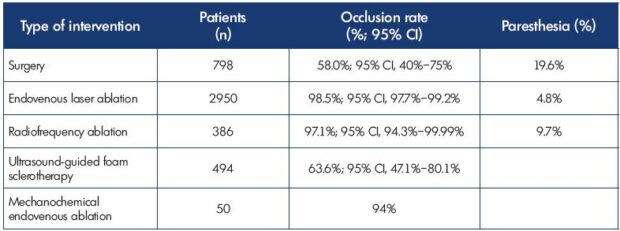
Table II. Treatment modalities for the small saphenous vein: occlusion rates and paresthesia.
Data from reference 19: Boersma D et al. J Endovasc Ther. 2016;23(1):199-211.
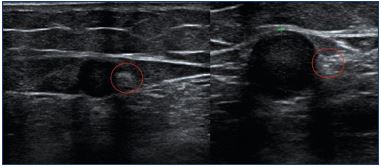
Using a 10 MHz ultrasound probe, Ricci20 showed that the sciatic nerve and its branches are easily identified in all cases into the popliteal fossa. Today, due to improvements in duplex ultrasound scanning and the high resolution of ultrasound probes (18 MHz), all the main nerves, including their branches up to 1 mm in thickness, are visible, and their entire path can be followed by ultrasonography. They are more easily visible in cross-section with their ultrasonic appearance being round and hyper-echogenic, in comparison with the surrounding tissues, and with their distinctive honeycomb pattern (Figure 4).18 Mapping the tibial nerve, fibular nerve, sural nerves, and the endings of the small saphenous vein prior to surgery or endothermal ablation can help the practitioner because there are numerous variations in the small saphenous vein endings (implantation level, aspect, and connection with the other veins that must be identified by ultrasound and reported) and divisions of the sciatic nerve (Figures 5, 6, and 7).
Into the popliteal fossa. When the small saphenous vein ends in the longitudinal axis (midline) of the limb, at the popliteal crease or above, the risk of damaging the nerve is very small, while the risk of damaging the nerve increases when the small saphenous vein ends above the popliteal crease and is displaced on the lateral aspect. There is a branch of the nerve (from the fibular nerve), which hooks onto the vein (Figure 8).
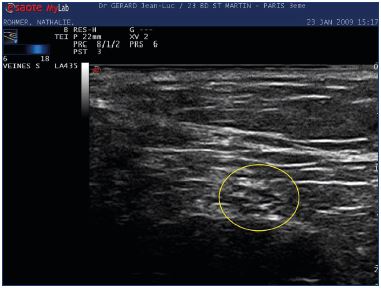
Figure 4. Cross-section of the tibial nerve using an 18 MHz
ultrasound probe that shows the classic honeycomb shape
(yellow circle).
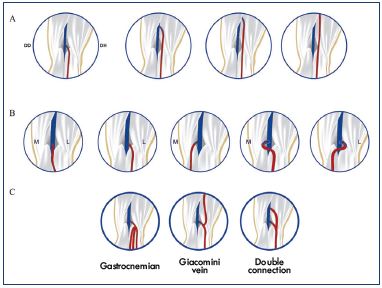
Figure 5. Variations in the small saphenous vein endings:
implantation levels (Panel A), aspect (Panel B), and connection
with other veins (Panel C).
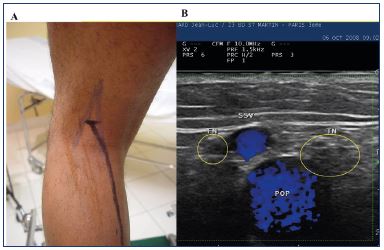
Figure 6. Panel A. mapping of the nerves (blue) and the small
saphenous vein (dark). Panel B. duplex image.
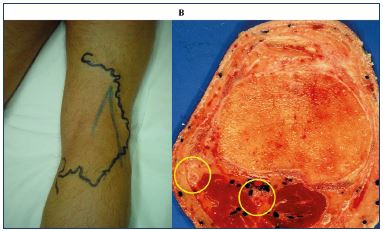
Figure 7. Panel A. mapping the nerves (blue) before phlebectomy
(tributary in black). Panel B. Anatomical view with fibular nerve
just under the skin.
From below the popliteal crease to the end of the calf. The sural nerve (lateral sural nerve) can join the small saphenous vein and be close to or in contact with the vein, at variable levels between the two layers of the aponeurosis (Figures 9 and 10). Fortunately, this situation is rare.
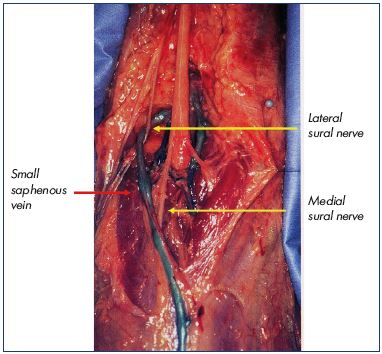
Figure 8. Ending of the small saphenous vein above the
popliteal crease, which is displaced on the lateral aspect due
to a branch of the nerve that hooks onto the vein.
Image courtesy of Prof Gillot.
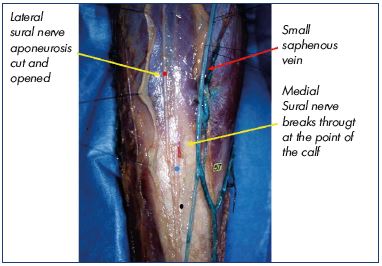
Figure 9. Medial sural companion of small saphenous vein
between the 2 layers of the aponeurosis.
Image courtesy of Prof Gillot.
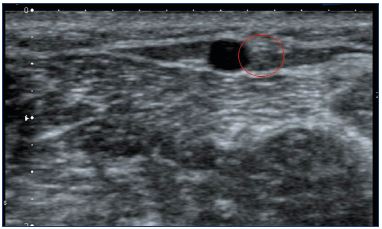
Figure 10. Duplex image of the sural nerve (red circle) close to
the small saphenous vein (between the 2 layers aponeurosis).
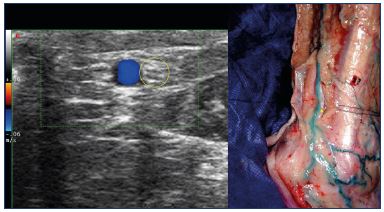
Figure 11. At the lateral malleolus: duplex image of the sural
nerve (left), a nerve that is in contact with small saphenous vein
(right – Image courtesy of Prof Gillot).
To the ankle. The nerve is always in contact with and possibly wrapped around the small saphenous vein (Figure 11). Ablation of the lowest part of the small saphenous vein should be avoided.
Isolating the nerve from the vein during tumescent anesthesia would prevent postoperative numbness at the lateral malleolus (Figures 12 and 13). In the event of an inability to separate the nerve from the vein (usually a segment <1 cm) due to a short endovenous laser ablation heating element (3 mm), it is possible to avoid treating these high-risk venous segments. When pain is felt, the generator pedal can be released, thereby immediately stopping the heat, like switching a light on or off. However, radiofrequency ablation of the small saphenous vein is more risky due to the length of the heating element (6.5 cm or 3.5 cm) and the impossibility of immediately stopping the heat (inertia), even after switching the device off when pain is felt, and it should be applied more cautiously. Therefore, the paresthesia rates are higher with radiofrequency ablation vs endovenous laser ablation, and the nerves may be damaged permanently. In some countries, treating the small saphenous vein by radiofrequency ablation is not allowed due to a lack of specific studies.
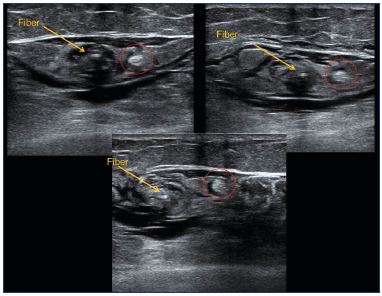
Figure 13. Sural nerve (red circle) pushed away from the small
saphenous vein by the tumescent anesthesia.
Energy according to the size of the vein
With endovenous laser ablation, the energy can be adjusted continuously in accordance with the diameter of the vein to be treated, ie, the linear endovenous energy density (LEED).21 We suggest a formula using 10 J/cm per diameter of the vein to be treated with a minimum of 60 J/cm. For example, if the vein diameter is 7 mm, then 70 J/cm should be applied. Laser energy is applied according to the information provided by duplex ultrasound mapping: increased for bulging veins or perforators and possibly decreased when proximal to the nerves. When treating larger veins, the energy needs to be increased, and, as the power should be invariable (10 watts maximum), the time must be increased.
Positioning the fiber at the ending of the small saphenous vein
During endovenous laser ablation, the positioning of the fiber tip needs to be very precise. The tip of the fiber has to be inserted into the small saphenous vein: (i) just below the saphenopopliteal junction if there are no tributaries; (ii) below the junction between the small saphenous vein and a competent vein: the Giacomini vein, the common trunk with a medial gastrocnemius vein or axial extension; and (iii) at any level in the thigh depending on the anatomy of the small saphenous vein thigh extension, just below the junction with a competent vein.
After endovenous laser ablation, especially when there has been an adequate delivery of energy and a successful procedure, there should be a significant shrinkage of the vein at the early duplex ultrasound examination. Duplex ultrasound can determine the success of the procedure by verifying that there is a definite reduction in vein size; the cockade image (plane roundel) or bagel image reflects the thickening of the intima (Figure 14) due to the heating, which matches the histologic image (Figure 15). Sick leave is not usually required and the patients can resume work in less than 2 days.
After ultrasound-guided foam sclerotherapy, duplex ultrasound images show a very slight reduction in vein size and very rarely a thickening of the intima. Consequently, there is a gradual reduction in the vein size; therefore, a long period of time is required for its disappearance (ie, 6 months [30% of cases], 1 year [63%], 18 months [80%], 2 years [85%]).22 No sick leave is usually required and work can be resumed in less than 2 days according to the literature.
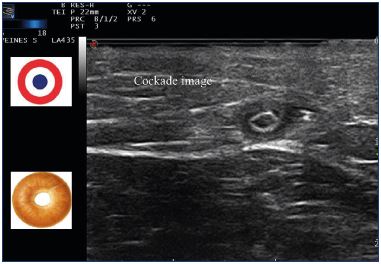
Figure 14. Cockade image: hypoechogenic image featuring
the vein lumen, an hyperechogenic one for the intima and
hypoechogenic one for the media-adventia or bagel image
after treatment with a 1470 nm endovenous laser and radial
fiber.
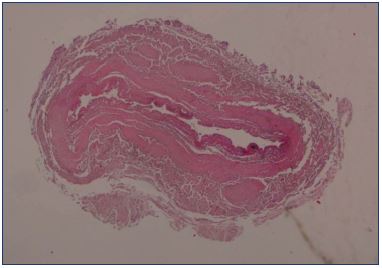
Figure 15. Histological examination of the great saphenous vein
after endovenous laser ablation using a 1470 nm laser and a
radial fiber.
Image courtesy of Dr Spreafico.
Randomized clinical trials
The two randomized clinical trials comparing endovenous laser ablation with conventional surgery for the small saphenous vein16,17 showed that abolishing the reflux of the small saphenous vein was significantly higher after endovenous laser ablation (96.2% and 91%) vs surgery (71.7% and 67%). Postoperative pain was significantly lower after endovenous laser ablation, allowing an earlier return to work. Minor sensory disturbances were significantly lower with endovenous laser ablation (7.5% and 6.5%) vs surgery (26.4% and 31%).
Case series
A meta-analysis on small saphenous vein treatment (Table II)19 showed that the highest occlusion rate (mean, 98.5%; 95% CI, 97.7%-99.2%) occurred with endovenous laser ablation (number of small saphenous veins =2950). Neurologic complications were most frequently reported after surgery (mean, 19.6%) vs thermal ablation (endovenous laser ablation: mean, 4.8%; radiofrequency ablation: mean, 9.7%). Deep venous thrombosis was a rare complication (0% to 1.2%).
Treating the small saphenous vein using other procedures
The incidence of recurrence from conventional surgery for small saphenous vein incompetence is high (up to 52% at 3 years),23 and conventional surgery is frequently associated with postoperative complications.24 Few studies give us the exact rate of paresthesia (26% to 28%)16,17,25 after small saphenous vein surgery. Indirect information has shown that small saphenous vein surgery is probably responsible for around half of the litigation claims related to vascular surgery. A study carried out by Markides et al26 from April 1995 to April 2007 identified 395 litigation claims that were related to vascular surgery. In terms of causes, 50% of the cases involved intraoperative problems, and, in ≈30% of these cases, varicose veins were involved. Nerve damage was the cause for complaint litigation claims in 36 cases. The fibular nerve was involved in 58%, the sural nerve in 6%, while, in 30% of cases, it was unclear which nerves were damaged. Thus, most of the litigation claims are due to small saphenous vein surgery, possibly because of the variable ending of the vein and its proximity to the nerves. Therefore, most of these claims could have been avoided if the position of the nerves (for the small saphenous vein and the tributaries) were marked preoperatively.
However, in a report by the CNAM (French public national health insurance)27 showed that 122 000 patients were treated with surgery for varicose veins in France in 2010; the cost was 264 million euros, and, on average, 26 days of sick leave were taken by 36% of the patients, costing 34 million euros. Although this report did not detail which veins have been operated, meaning that it was not specific to the small saphenous vein, the number of days of sick leave is the same regardless of the varicose veins treated.
Traditional surgery has been graded 2B, according to the American guidelines28 and 2A according to the European Venous Forum guidelines.29 The NICE guidelines30 propose surgery only if thermal ablation and ultrasound-guided foam sclerotherapy are unsuitable.
Therefore, treating the small saphenous vein with surgery should be the very last option due to the high incidence of recurrence and paresthesia and the excessive number of days of sick leave and fees for the nursing services required. Although open surgery provides good results in competent hands, it is no longer the gold-standard treatment for small saphenous vein incompetency. According to evidence based medicine,31 surgery is reserved for certain patients depending on the circumstances, the patients themselves, or their social (economic) problems.
Sclerotherapy that is performed in a doctor’s office is the easiest and cheapest procedure for varicose veins.
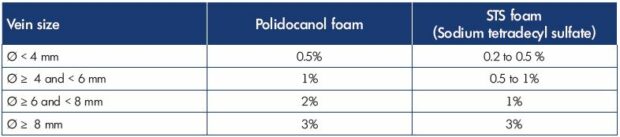
Table III Algorithm for treating the small saphenous vein with sclerotherapy. The maximum volume of foam per session is 10 mL.
Ref. 36. Hamel-Desnos C. Echo-doppler per procédure: sclérothérapie à la mousse.
In Guex JJ, Hamel-Desnos C, eds. Ultrasons et Phlébologie. Editions Phlébologiques Françaises-Paris; 2016:109-121
Ultrasound-guided sclerotherapy was first described at the end of the 1980s,32,33 and it provided better results with improvements in safety and precision. This procedure was improved with ultrasound-guided foam sclerotherapy,34,35 which provided better efficacy (fewer injections and fewer sessions) compared with liquid sclerotherapy. Nevertheless, to provide optimal results, ultrasound-guided foam sclerotherapy must be performed with an adequate sclerosing agent and at the right concentration and volume (Table III). Certainly, ultrasound-guided foam sclerotherapy is a standardized procedure, but, to do this, certain rules must be respected regarding efficacy and safety.36 First, the mixture of the sclerosing agent and gas, which is done with a three-way tap or female-female biconnectors, must use 1 volume of sclerosing agent to 4 volumes of gas (room air-filtered or carbon dioxide or a mixture of carbon dioxide and oxygen). Second, a treatment algorithm should be adopted to adjust the doses according to the vein diameter. Third, for safety reasons, ultrasound-guided foam sclerotherapy must be carried out entirely under ultrasound control to monitor: (i) location of the vein to be injected and detect possible nearby arteries; (ii) vein puncture; (iii) needle position check; (iv) sclerosing injection; (v) post injection check; and (vi) evaluation of vein spasm and vein filling.
The major complications of sclerotherapy occur by mistakenly injecting the artery companion to the small saphenous vein, which can cause large cutaneous necrosis, or even worse, muscular necrosis. There are no rules (no exact anatomical locations) that can determine the exact position of the arteries; therefore, it is mandatory to use duplex ultrasound to identify a safe zone without arteries before injection. The main advantage of the procedure is the rarity of paresthesia; however, when it occurs, it is probably due to excessive compression by bandages. The use of compression stockings after sclerotherapy, which is often recommended, has never been shown to offer any advantages; therefore, they are deemed useless and possibly deleterious.
If the diameter of the vein is <5 mm, sclerotherapy could be recommended. For veins between 5 and 6 mm, sclerotherapy could be balanced with thermal ablation. However, when the small saphenous vein is too large in diameter, the amount of sclerotherapy solution that needs to be injected into the patient could be beyond the safety recommendations (maximum of 10 mL of foam per session),37 which could be less efficient during short- or long-term follow-up. In fact, the main disadvantage of ultrasound guided foam sclerotherapy is high recanalization rates of veins >6 mm in diameter, which often necessitates at least a second treatment. Chemical ablation is, most of the time, not a one-go treatment, as it often requires several sessions. In addition, there are higher risks of phlebitis (inflammatory area) and brown staining of the skin when veins are superficial (tributaries).
There are no specific studies on treating the small saphenous vein with cyanoacrylate glue or mechanochemical endovenous ablation and very few patients have been treated with these methods; therefore, we cannot give recommendations.
Conclusion
All the wavelengths and optical fibers have shown a high rate of success, but a 1470 nm diode laser and protected fiber (radial fiber) offer a high rate of success with the lowest number of side effects. Endovenous laser ablation is adapted to the small saphenous vein, regardless of its size, ending, or anatomy, on the condition that the small saphenous vein and nerves are mapped prior to the procedure. High-frequency ultrasound probes (18 MHz or at least 14 MHz) make the procedure easier, and these probes should be used widely to avoid nerve damage, which will consequently decrease the number of litigation claims. Adapted material and training in ultrasonography are indispensable for achieving this goal. Even if endovenous laser ablation seems to be a safe, less traumatizing, and efficient technique, the choice of a technique actually depends on the preferences of the patient (economic reasons, reimbursement, knowledge of the technique, age, etc) and the abilities of the practitioner to use one or more of the other techniques.

REFERENCES
1. Society of Great Britain and Ireland. Eur J Vasc Endovasc Surg. 2004;28(4):400- 403.
2. Engelhorn CA, Engelhorn AL, Cassou MF, Salles-Cunha SX. Patterns of saphenous reflux in women with primary varicose veins. J Vasc Surg. 2005;41(4):645-651.
3. Proebstle TM, Gül D, Kargl A, Knop J. Endovenous laser treatment of the lesser saphenous vein with a 940-nm diode laser: early results. Dermatol Surg. 2003;29(4):357-361.
4. Ravi R, Rodriguez-Lopez JA, Trayler EA, Barrett DA, Ramaiah V, Diethrich EB. Endovenous ablation of incompetent saphenous veins: a large singlecenter experience. J Endovasc Ther. 2006;13(2):244-248.
5. Theivacumar NS, Beale RJ, Mavor AI, Gough MJ. Initial experience in endovenous laser ablation (EVLA) of varicose veins due to small saphenous vein reflux. Eur J Vasc Endovasc Surg. 2007;33(5):614-618.
6. Gibson KD, Ferris BL, Polissar N, Neradilek B, Pepper D. Endovenous laser treatment of the small [corrected] saphenous vein: efficacy and complications. J Vasc Surg. 2007;45(4):795-801.
7. Park SJ, Yim SB, Cha DW, Kim SC, Lee SH. Endovenous laser treatment of the small saphenous vein with a 980-nm diode laser: early results. Dermatol Surg. 2008;34(4):517-524.
8. Nwaejike N, Srodon PD, Kyriakides C. Endovenous laser ablation for short saphenous vein incompetence. Ann Vasc Surg. 2009;23(1):39-42.
9. Park SW, Hwang JJ, Yun IJ, et al. Endovenous laser ablation of the incompetent small saphenous vein with a 980-nm diode laser: our experience with 3 years follow-up. Eur J Vasc Endovasc Surg. 2008;36(6):738-742.
10. Kontothanassis D, Di Mitri R, Ferrari Ruffino S, et al. Endovenous laser treatment of the small saphenous vein. J Vasc Surg. 2009;49(4):973-979.
11. Trip-Hoving M, Verheul JC, van Sterkenburg SM, de Vries WR, Reijnen MM. Endovenous laser therapy of the small saphenous vein: patient satisfaction and short-term results. Photomed Laser Surg. 2009;27(4):655- 658.
12. Huisman LC, Bruins RM, van den Berg M, Hissink RJ. Endovenous laser ablation of the small saphenous vein: prospective analysis of 150 patients, a cohort study. Eur J Vasc Endovasc Surg. 2009;38(2):199-202.
13. Desmyttère J, Grard C, Stalnikiewicz G, Wassmer B, Mordon S. Endovenous laser ablation (980 nm) of the small saphenous vein in a series of 147 limbs with a 3-year follow-up. Eur J Vasc Endovasc Surg. 2010;39(1):99-103.
14. D’Othée JB, Walker TG, Kalva SP, Ganguli S, Davison B. Endovenous laser ablation of the small saphenous vein sparing the saphenopopliteal junction. Cardiovasc Intervent Radiol. 2010;33(4):766-771.
15. Doganci S, Yildirim V, Demirkilic U. Does puncture site affect the rate of nerve injuries following endovenous laser ablation of the small saphenous veins? Eur J Vasc Endovasc Surg. 2011;41(3):400-405.
16. Samuel N, Carradice D, Wallace T, Mekako A, Hatfield J, Chetter I. Randomized clinical trial of endovenous laser ablation versus conventional surgery for small saphenous varicose veins. Ann Surg. 2013;257(3):419-426.
17. Roopram AD, Lind MY, Van Breussel JP, et al. Endovenous laser ablation versus conventional surgery in the treatment of small saphenous vein incompetence. J Vasc Surg Venous Lymphat Disord. 2013;1(4):357-363.
18. Gerard JL. What is new in laser treatment of the saphenous and perforating veins. In Gloviczki P, Shields R, Bjarnason H, Becquemin JP, Gloviczki M, eds. Mayo Clinic International Vascular Symposium 2011: Advances and Controversies in Vascular Medicine, Vascular Surgery and Endovascular Interventions. Edizioni Minerva Medica. 2011:349-354.
19. Boersma D, Kornmann VN, van Eekeren RR, et al. Treatment modalities for small saphenous vein insufficiency: systematic review and meta-analysis. J Endovasc Ther. 2016;23(1):199-211.
20. Ricci S. Ultrasound observation of the sciatic nerve and its branches at the popliteal fossa: always visible, never seen. Eur J Vasc Endovasc Surg. 2005;30(6):659-663.
21. Proebstle TM, Moehler T, Gül D, Herdemann S. Endovenous treatment of the great saphenous vein using a 1,320 nm Nd:YAG laser causes fewer side effects than using a 940 nm diode laser. Dermatol Surg. 2005;31(12):1678- 1683.
22. Hamel-Desnos C, Ouvry P, Benigni JP, et al. Comparison of 1% and 3% polidocanol foam in ultrasound guided sclerotherapy of the great saphenous vein: a randomised, double-blind trial with 2 year-follow-up. “The 3/1 Study.” Eur J Vasc Endovasc Surg. 2007;34(6):723-729.
23. van Rij AM, Jiang P, Solomon C, Christie RA, Hill GB. Recurrence after varicose vein surgery: a prospective long-term clinical study with duplex ultrasound scanning and air plethysmography. J Vasc Surg. 2003;38(5):935-943.
24. Rashid HI, Ajeel A, Tyrrell MR Persistent popliteal fossa reflux following saphenopopliteal disconnection. Br J Surg. 2002;89(6):748-751.
25. O’Hare JL, Vandenbroeck CP, Whitman B, et al. A prospective evaluation of the outcome after small saphenous varicose vein surgery with one-year follow-up. J Vasc Surg. 2008;48(3):669-673.
26. Markides GA, Subar D, Al-Khaffaf H. Litigation claims in vascular surgery in the United Kingdom’s NHS. Eur J Vasc Endovasc Surg. 2008;36(4):452-457.
27. Rapport de l’assurance maladie sur les charges et produits pour l’année 2013. Constats https://www.ameli.fr/ fileadmin/user_upload/documents/ cnamts_rapport_charges_produits_2013. pdf.
28. Gloviczki P, Comerota AJ, Dalsing MC, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(suppl 5):2S-48S.
29. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. Int Angiol. 2014;33(2):87- 208.
30. National Institute for Health and Care Excellence. Varicose veins: diagnosis and management. http://guidance.nice. org.uk/cg168. Published July 24, 2013. Accessed July 19, 2017.
31. Guyatt G, Gutterman D, Baumann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American college of chest physicians task force. Chest. 2006;129:174-181.
32. Schadek M. Doppler et échotomographie dans la sclérose des veines saphènes. Phlébologie. 1986;39:697-716.
33. Knight RM, Vin F, Zygmunt JA. Ultrasonic guidance of injections into the superficial venous system. In: Davy A, Stemmer R, eds. Phlébologie 1989. John Libbey Eurotext; 1989:339-341.
34. Cabrera J, Cabrera García-Olmedo JR. Nuevo metodo de esclerosis en las varices tronculares. Patol Vascular. 1995;4:55-73.
35. Monfreux A. Traitement sclérosant des troncs saphèniens et leurs collatérales de gros calibres par la méthode MUS. Phlébologie. 1997;50:351-353. 36. Hamel-Desnos C. Echo-doppler per procédure: sclérothérapie à la mousse. In Guex JJ, Hamel-Desnos C, eds. Ultrasons et Phlébologie. Editions Phlébologiques Françaises-Paris; 2016:109-121. 37. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29(6):338-354.