Chronic venous disorders: pharmacological and clinical aspects of micronized purified flavonoid fraction
pharmacological and clinical
aspects of micronized purified
flavonoid fraction
92284 Suresnes, France
Abstract
Micronized purified flavonoid fraction (MPFF) is a flavonoid-based venoactive drug that is more potent than pure diosmin due to the presence of additional flavonoids, such as hesperidin, diosmetin, linarin, and isorhoifolin. In addition, the dissolution and absorption rates of MPFF increase due to the micronization of its active ingredients. The micronization process improves exposure to MPFF– derived metabolites that are responsible for its pharmacological activity. The positive impact of micronization on the pharmacological activity of purified flavonoid fraction has been demonstrated in both animal and clinical pharmacological trials.
MPFF improves venous tone by modulating noradrenergic signaling and reducing norepinephrine metabolism and MPFF also protects against inflammation-related valve damage by inhibiting the leukocyte-endothelium interaction, decreasing capillary permeability, improving capillary resistance, and increasing lymphatic drainage. The best dose-effect ratio is achieved with 1000 mg.
MPFF is an important treatment option for chronic venous disorders because it relieves symptoms at all stages, significantly alleviates venous edema, and, in more advanced stages, MPFF may be used in conjunction with sclerotherapy, surgery, and/or compression therapy for patients undergoing stripping or an endovenous operation for varicose vein ablation. MPFF may also be used as an adjunctive therapy in patients with active venous ulcers, especially in patients with chronic large ulcers.
Introduction
Flavonoids are one of the main active phytoconstituents found in plant extracts and the micronized purified flavonoid fraction (MPFF*) is included in this group. The use of plants and isolated phytochemicals for the prevention and treatment of various health ailments has been in practice for years. About 25% of the drugs prescribed worldwide are derived from plants and 121 such active compounds are currently in use. In addition, 11% of the 252 drugs on the World Health Organization (WHO)’s essential medicine list are exclusively plant based.1
Besides providing pigmentation, flavonoids play an important role in the growth and development of plants, such as protecting against UVB radiation, fungal infection, and microbial and insect attacks. Flavonoids have been reported to chelate metal, inhibit enzymes, inhibit cellular proliferation, induce apoptosis, stabilize membranes, and scavenge free radicals. Flavonoids have antioxidant, anti-inflammatory, antiallergic, antibacterial, osteogenic, cytotoxic, antitumoral, hepatoprotective, antithrombotic, and antiviral pharmacological properties.2
MPFF consists of diosmin (90%) and an additional flavonoid fraction (ie, diosmetin, hesperidin, linarin, and isorhoifolin; 10%) and it is widely used to treat symptoms related to chronic venous disorders and hemorrhoidal disease. This review contains an overview of the pharmacological activities and clinical benefits of MPFF on chronic venous disorders.
MPFF Chemistry
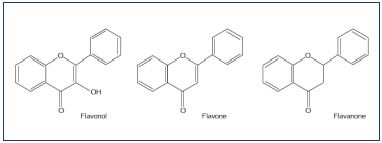
Flavonoids are a class of low molecular weight, secondary plant phenolics with significant antioxidant and chelating properties and they are characterized by a flavin nucleus and an oxygenated heterocyclic skeleton that is composed of two aromatic rings. Substitutions at different positions in the ring lead to various types of flavonoid compounds, including flavone, flavonol, and flavonone (Figure 1). To date, more than 4000 flavonoids have been identified and they are widely distributed in the leaves, seeds, bark, and flowers of plants that constitute an integral part of the human diet. The most important groups are the anthocyanidins, catechins, flavones, flavanones, and flavonols (Table I).

Figure 2. Harvest of small immature fruits (10 to 20 mm in
diameter) to produce MPFF.
The fruit are harvested when they fall from the tree at the
end of the flowering period. The sun-dried oranges are then
ground and hesperidin is extracted in powder form.
The MPFF components diosmin, diosmetin, linarin, and isorhoifolin are synthesized from hesperidin, which is extracted from Citrus aurantium var amara, a type of small, “bitter,” immature orange (Figure 2). Diosmin and its aglycone diosmetin (3’, 5, 7-trihydroxy-4’-methoxyflavone) belong to the flavonol and flavone groups, while hesperidin, which differs from diosmin by the absence of a double bond between two carbon atoms, is part of the flavanone group (Table I). These compounds also occur naturally in citrus fruit. Both linarin (Acacetin 7-rutinoside) and isorhoifolin are derived from flavones.
Pharmaceutical characteristics of MPFF
MPFF excipients
The excipients included in the MPFF-based drug composition are organic with a mineral, animal, or plant origin and they are known to be safe and well tolerated. The main excipients used in MPFF include microcrystalline cellulose, sodium starch glycolate, gelatin, magnesium stearate, and talc. They are used for the following reasons:
1. Microcrystalline cellulose is an inert substance that is widely used as a binder and diluent in many pills and tablets.3 As an insoluble fiber, microcrystalline cellulose is not absorbed into the blood stream, so it cannot cause toxicity when taken orally, and as a result, it is often used as a placebo in controlled drug studies. Microcrystalline cellulose has no impact on the dissolution rate of any active ingredients; consequently, it cannot improve their absorption and cannot replace the benefits of the micronization
2. Sodium starch glycolate is derived from potato starch, is not contraindicated for celiac disease, and is a disintegrant.
3. Gelatine binds to the active molecules. It has a bovine, ovine, or poultry origin; therefore, it is compatible with the Muslim religion and a gout diet.
4. Magnesium stearate and talc are inert substances used as lubricants.
MPFF active ingredients
Contrary to “pure diosmins,” such as Phlebodia®, MPFF includes diosmin (90%) and additional flavonoids (ie, diosmetin, linarin, isorhoifolin, and hesperidin) expressed as hesperidin (10%). Each flavonoid present in MPFF contributes to its pharmacological effect. In a hamster model of venous inflammation, where leaky sites are formed in the cheek pouch, each of these additional flavonoids administered separately displayed an antileakage effect comparable to or greater than diosmin.4 These results illustrate that MPFF is more potent than pure diosmin and that each of the flavonoid substances present in the MPFF composition contributes to its action (Figure 3). In a related article, Paysant et al concluded that “it should be stressed that MPFF decreases the appearance of leaky sites more than any of its single constituents, which is most likely explained by the synergistic action of all the flavonoids present in its formulation.”4
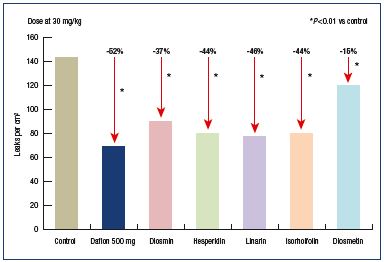
Figure 3. Effect of oral administration of MPFF or diosmin alone
on permeability induced by ischemia and reperfusion.
Modified from reference 4: Paysant J et al. Int Angiol. 2008;27:81-85.
Pharmacokinetics of MPFF
There are no known drug interactions with MPFF since marketing authorization.5
Absorption and distribution of the MPFF active ingredients
Diosmin is a Biopharmaceutical Classification System IV (BCS IV) compound, which means that it has low solubility and low permeability.6 Diosmin is not directly absorbed by the body, and, as shown in studies, it is not found in the circulation after oral administration. Metabolism studies showed that diosmin is metabolized by gut microbiota within the gastrointestinal tract to produce several metabolites that are then further absorbed.7 However, nonmetabolized diosmetin is not found in the circulation; therefore, it is not the active compound responsible for the venotonic action following oral administration of diosmin. Other metabolites of MPFF, such as glucuronide derivatives of diosmetin, and other metabolic breakdown products (phenolic acid derivatives) have been identified in the circulation and/or urine.8
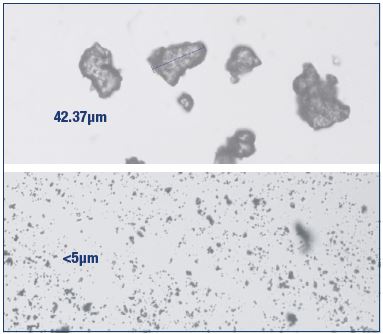
Figure 4. Nonmicronized and micronized purified flavonoid
fractions.
Nonmicronized purified flavonoid fraction (top) and MPFF
(bottom). The micronization process increases the bioavailability
of the flavonoids comprised in the MPFF composition.
Micronization enhances MPFF absorption
Micronization is achieved by using air jets operating at near supersonic velocities to create repeated particle-on-particle collisions that result in an average particle size that is Figure 4). Absorption of compounds derived from diosmin metabolism, measured by the urinary excretion of total radioactivity following oral administration of 14C-diosmin in humans, was significantly (P=0.0004, analysis of variance) improved with micronization (57.9}20.2%) vs nonmicronization (32.7}18.8%).9 Micronization increases the dissolution rate of diosmin and enhances its metabolism, which in turn improves exposure to the metabolites that are responsible for its pharmacological activity.
The positive impact of micronization on the pharmacological activity of purified flavonoid fraction has been demonstrated in both preclinical and clinical pharmacological trials. In a study performed in hamsters, MPFF reduced the ischemia/ reperfusion-induced macromolecular permeability in the cheek pouch microcirculation to a greater extent than the nonmicronized purified flavonoid fraction (83.4% vs 47.9%, respectively).10 In a former clinical study, 500 mg of MPFF taken twice daily for 2 months improved clinical symptoms and decreased venous outflow parameters more than 300 mg of nonmicronized diosmin taken thrice daily (900 mg dialy).11 Therefore, micronization is essential for effective absorption of the active compounds.
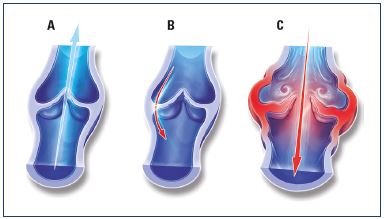
Figure 5. Illustrations of venous valves with and without reflux.
Illustrations of a normal venous valve without reflux (Panel A), a
valve with a nonpathological commissural reflux usually seen in
the evening after being in a prolonged upright position (Panel
B), and a valve with a pathological intervalvular reflux (Panel C).
From reference 24: Tsoukanov Y et al. Phlebolymphology. 2015;22:18-24.
Image courtesy of the author.
Metabolism and elimination of the MPFF metabolites
In humans, elimination of micronized diosmin is relatively rapid, with 34% of the 14C-labeled diosmin being excreted in urine and feces over the first 24 hours and 86% over the first 48 hours, with a 100% cumulative excretion of the dose in urine and feces after 168 hours (109}23%).9 Similarly, the other citrus flavanone aglycones, such as hesperetin and naringenin, are recovered in plasma as their conjugated forms and are subsequently excreted in urine.12-15
Pharmacological effects of MPFF
MPFF activity on venous tone
Traditionally, venous hypertension, which underlies all clinical manifestations of chronic venous disease, was thought to result primarily from valvular incompetence related to excessive venous dilation due to a weakness in the vein wall and/or low venous tone. Consequently, much of the earlier research on MPFF was centered on its effects on venous tone. Treating patients with MPFF, two 500 mg tablets daily, reduced venous distension and venous capacitance and improved venous tone in women with various grades of venous insufficiency, ie, healthy women, women with venous insufficiency related to postthrombotic syndrome, or pregnant women.16 In a another trial, MPFF, two 500 mg tablets daily, improved venous tone in female volunteers with abnormal venous elasticity and a high risk of developing varicose veins.17 MPFF acts on venous tone by modulating noradrenergic signaling and reducing norepinephrine metabolism.18
Antioxidant properties of MPFF
MPFF inhibits oxygenated free radical production in vitro in zymosan-stimulated human neutrophils, rat leukocytes, and mouse macrophages. Additional trials demonstrated that MPFF leads to the following: (i) normalization of prostaglandin E2 or F2 and thromboxane B2 synthesis in inflammatory granulomas in rats; (ii) reduction in the bradykinin- or ischemia-induced microvascular permeability in rat cremaster muscle; (iii) reduction in the histamine-, bradykinin-, leukotriene B4-induced ischemia and reperfusion or oxidant challenge in the hamster cheek pouch; (iv) protection of endothelial cells from lipid peroxidation in bovine aortic endothelial cells and human skin fibroblasts.7,19
Leukocyte activation and adhesion
In the last 10 years, research focus has shifted toward determining the action of venoactive drugs on chronic inflammatory processes affecting large and small venous vessels and valves. Such inflammatory processes start with inappropriate activation of leukocytes in the veins. Former pharmacological studies in animals have demonstrated that MPFF inhibits venous inflammation by reducing leukocyte rolling, adhesion, and migration in rats, by decreasing the number of parenchymal dead cells after venular mesenteric occlusion in rats, and by reducing leukocyte adhesion and/or migration after ischemia-reperfusion injury in hamster skinfold or rat skeletal muscle. In clinical studies, MPFF reduced the expression of monocyte or neutrophil CD62L and the endothelial activation markers intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) on human leukocytes from patients with venous ulcers.7,19
Protective effect against inflammation-related valve damage in chronic venous disorders
Pharmacological studies have shown that MPFF mitigates or blocks the effects of chronic inflammation in the microand macrocirculation. In a model of venous occlusion and reperfusion, elevation of venous blood pressure increased inflammation and tissue injury.20 In MPFF-treated animals, markers of inflammation decreased in a dose-dependent manner. MPFF also significantly reduced parenchymal cell death, leukocyte rolling, adhesion to postcapillary venules, and migration.21 In rats with venous hypertension induced by creating an arteriovenous fistula, Takase et al showed that MPFF treatment resulted in a significant, dose-dependent reduction in the reflux rate in rats with higher than normal venous hypertension, demonstrating the protective effects of MPFF on the macrocirculation.22
By delaying or blocking the inflammatory reaction in venous valves and walls, these data suggest that MPFF may delay the development of venous reflux and suppress damage to valve structures in a rat model of venous hypertension. These observations were confirmed in a new study using the same animal model. MPFF reduced edema and fistula blood flow produced by an acute arteriovenous fistula and reduced granulocyte and macrophage infiltration into the valves, similarly to the previous study.23
In clinical trials, 1000 mg/day of MPFF for 2 months eliminated the transitory commissural reflux observed in patients presenting with subjective leg symptoms without visible signs of chronic venous disorders; these patients are categorized as C0s according to the clinical, etiological, anatomical, and pathophysiological (CEAP) classification system (Figure 5).24 Transitory reflux elimination was paralleled with pain relief and an improvement in quality of life. In this trial, consecutive C0s patients were enrolled and assessed for symptom intensity using the visual analog scale (VAS), quality of life using the ChronIc Venous Insufficiency quality of life Questionnaire (CIVIQ-20), and saphenous reflux duration and saphenous vein diameter using a twice-daily Duplex scan examination (morning and evening). A total of 41 C0s patients were enrolled, and, of these patients, 15 had no reflux in either the morning or evening and 26 had transitory evening reflux with 22 being commissural and 4 intervalvular. The saphenous vein diameter was greater in the subgroup of patients with transitory reflux compared with patients without reflux (P<0.05). After MPFF treatment, there was a trend toward a reduction in intervalvular reflux length (despite being nonsignificant), while transitory commissural refluxes (n=22) no longer appeared. Additionally, vein diameter returned to normal. These results mirror the protective effect of MPFF on venous valve structures in humans.
Capillary permeability and resistance
MPFF decreases the volume of induced edema in the rat paw and improves microvascular reactivity and functional capillary density after ischemia and reperfusion in the hamster cheek pouch. In humans, MPFF significantly improved capillary hyperpermeability compared with placebo in patients with idiopathic cyclic edema,25 decreased the abnormal capillary filtration rate in patients with chronic venous insufficiency as evaluated using strain gauge plethysmography, and improved capillary resistance significantly compared with placebo in patients with abnormal capillary fragility.26
Lymphatics
MPFF increased the contractility of mesenteric lymphatic collecting ducts in sheep, increased the frequency of spontaneous contractions in bovine mesenteric lymphatics, and improved lymphatic drainage in sheep and dogs. In clinical pharmacology, MPFF decreased intralymphatic pressure and increased the number of functional lymphatic capillaries, which resulted in an improvement in lymphatic drainage in patients suffering from skin changes.27-29
Dose-effect ratio for MPFF
Contrary to the statement that the administration of 600 mg of diosmin once daily is sufficient, Amiel et al reported that the best dose-effect ratio is achieved with 1000 mg of MPFF, which means at least 900 mg of diosmin.30 No significant differences were found with the single MPFF tablet dosing; on the other hand, after administration of two or four tablets of MPFF 500 mg, legs with residual postphlebitic abnormalities showed significant improvements in venous capacitance, venous distensibility during occlusion at 40, 50, and 60 mm Hg, and total venous emptying time and its longest component (ie, time needed to empty the last 50%) compared with contralateral healthy legs. There was a linear relationship between the logarithm of the MPFF dose and the effect on venous hemodynamics in both abnormal and normal legs. For most measurements, the results obtained with four tablets were significantly reinforced compared with those obtained with two tablets, but the effect was not doubled. Definitely, the best dose-effect ratio was achieved with two tablets of MPFF 500 mg on the hemodynamic parameters previously described. Therefore, a single dose of 600 mg of diosmin is probably insufficient.
Safety of MPFF
In a study in rats, when MPFF was administered by gastric intubation for 26 weeks, no deaths, changes in weight, or abnormalities of standard functional tests were observed.31 In a study in humans, MPFF administration resulted in minor side effects in only 10% of the subjects compared with 13.9% of those treated with placebo.32 Adverse events were similar in nature and incidence between these patient groups. The rate of discontinuation due to adverse events (primarily of gastrointestinal origin) was comparable among patients receiving two tablets of MPFF 500 mg daily or placebo (1.1 vs 3.2%). In this analysis, the incidence of adverse events was not significantly different in patients >70 years old or with concomitant diseases (ie, hypertension, atherosclerosis, diabetes mellitus, neurological/psychiatric disease, or alcoholism) than the total population group.32 In addition, MPFF did not appear to interact with the drugs used to treat these concomitant diseases. The incidence of adverse events did not increase with long-term treatment with two tablets of MPFF 500 mg daily.33 Treatment with MPFF did not modify blood pressure or laboratory parameters. Systolic or diastolic blood pressure and laboratory values did not change during treatment with two tablets of MPFF 500 mg daily for 1 year in a clinical trial that monitored these parameters every 4 months.33 Laboratory values (eg, red blood cells, leukocytes, hemoglobin, hepatic enzymes, blood urea, blood glucose and lipids, and creatinine) remained within normal physiological ranges.
Role of MPFF in the treatment of chronic venous disorders
Venous symptoms
MPFF plays a role in the management of symptomatic patients at the earliest stages of chronic venous disease, given that compression therapy may be the only other appropriate form of therapy for such patients. However, due to poor compliance with compression therapy in certain countries,34,35 pharmacological treatment with venoactive drugs (including MPFF) may be the only available alternative. Rabe et al showed that approximately 20% of all patients consulting their general practitioner for any reason could be assigned to class C0s; therefore, it is important to treat these patients effectively.36 Studies of venoactive drugs on this specific C0s patient are not yet available.
Despite a lack of homogeneity between studies, a Cochrane review of 44 controlled studies of venoactive drugs vs placebo37 showed significant treatment benefits of the venoactive drugs compared with placebo for pain, cramps, heaviness, sensation of swelling, and paresthesia (Table II). The only nonsignificant effects were for itching, but the sample size was the lower (n<500). The placebo effect in these studies is far from being insignificant and thus large samples are needed to observe any treatment effect on venous symptoms. Sample sizes in Table II are over 1000 patients for most variables.
In a recent double-blind, placebo-controlled trial including 592 symptomatic patients (leg pain and heaviness) randomly allocated to either MPFF treatment (n=296; 1000 mg/day for 4 months) or placebo (n=296; same process), symptom intensity as assessed using a 10 cm-visual analog scale decreased from 6.2±1.5 cm to 3.4±2.4 cm after 4 months of MPFF treatment (vs 6.0±1.4 cm to 3.7±2.5 cm in the placebo group; P=0.031). In addition, the CIVIQ quality of life questionnaire scores increased from 57.3±19.3 to 69.9±20.6 points in the MPFF treatment group (vs 59.5±17.9 to 69.1±20.6 points in the placebo group; P=0.040).38 Between group differences favored MPFF for both symptom relief and quality of life improvement (Figure 6). MPFF enhances quality of life by relieving symptoms right from the very beginning (C0s) and at all stages of the disease.
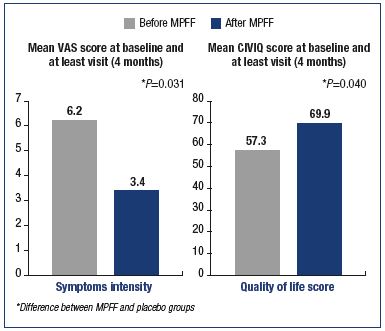
Figure 6. Benefits of the micronized purified flavonoid fraction on symptoms and quality of life of C3 and C4 patients. Modified from reference 38: Rabe E et al. Int Angiol. 2015;34:428-436.
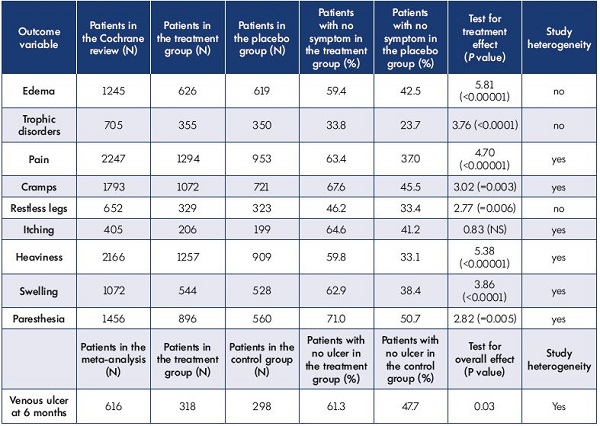
Table II. Global results of combined analyses for all venoactive drugs.
All outcomes were analyzed as a percentage of improved patients.
Adapted from the Cochrane review of phlebotonics for venous insufficiency37 and the meta-analysis of adjunctive MPFF on venous ulcers.43
Venous edema
Although nonspecific, edema is one of the most frequent and typical signs of chronic venous disease. All other causes of edema should be excluded to confirm its venous origin. Venous edema is described as sporadic, unilateral or bilateral, and more frequently located at the ankle. Several well-conducted controlled trials vs placebo or compression stockings have shown a reduction in edema by oral venoactive drugs, such as MPFF.18 The analysis of a pool of 1245 patients from the Cochrane review showed significant benefit of such drugs in alleviating edema (Table II).37
In a meta-analysis of ten publications of randomized controlled trials comparing venoactive drugs with either a placebo or another venoactive drug (hydroxyethylrutoside, ruscus extracts, and diosmin) on the reduction in ankle circumferences in 1010 patients complaining of venous edema at any CEAP stage, the mean reduction in ankle circumference was significantly greater with MPFF than with any other venoactive drug (P<0.0001; Figure 7). In addition, results for diosmin were not significant compared with placebo.34 MPFF significantly alleviates patients from edema vs other venoactive drugs.
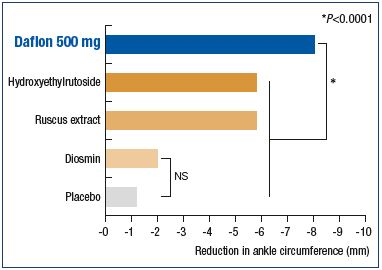
Figure 7. Superiority of the MPFF over placebo and other
venoactive drugs in relieving venous edema.
Modified from reference 39: Allaert FA. Int Angiol. 2012;31:310-315.
More advanced stages of chronic venous disease (C2 to C6 patients)
In more advanced stages of chronic venous disease, MPFF may be used in conjunction with sclerotherapy, surgery, and/or compression therapy in patients undergo stripping35,36 or an endovenous operation for varicose vein ablation.37 MPFF may be considered an adjunctive therapy in patients with active venous ulcers, especially in those with chronic large ulcers.43
Conclusion
The availability of multiple methods to treat chronic venous disorders necessitates a clinical evidence-based ranking to better inform and satisfactorily treat patients. An ideal treatment would rapidly and significantly reduce symptoms, stop disease progression, act on all components of the disease, protect against complications, remain effective in the long term, and be well tolerated. The aim is to improve patient’s quality of life as quickly as possible.
MPFF is the only venoactive drug to demonstrate significant anti-inflammatory and venoprotective actions, which distinguishes this drug from other venoactive drugs to provide patients with rapid and substantial relief of symptoms. MPFF also provides a unique protection against complications by preserving the venous valves and walls. These facts have been recognized by systematic reviews7,44 and both national and international guidelines,18,45 where MPFF has the highest level of recommendation as a firstline treatment for the management of chronic venous disorder–related symptoms and edema at all stages and as an adjunctive therapy for venous ulcers.
REFERENCES
1. Patel K, Gadewar M, Tahilyani V, Patel DK. A review on pharmacological and analytical aspects of diosgenin: a concise report. Nat Prod Bioprospect. 2012;2:46- 52.
2. Patel K, Gadewar M, Tahilyani V, Patel DK. A review on pharmacological and analytical aspects of diosmetin: a concise report. Chin J Integr Med. 2013;19:792- 800.
3. FDA’s SCOGS database. Cellulose and microcrystalline cellulose. http://www.fda.gov/Food/ IngredientsPackagingLabeling/GRAS/ SCOGS/ucm261287.htm. Accessed February 23, 2016.
4. Paysant J, Sansilvestri-Morel P, Bouskela E, Verbeuren TJ. Different flavonoids present in the micronized purified flavonoid fraction (MPFF at a dose of 500 mg) contribute to its anti-hyperpermeability effect in the hamster cheek pouch microcirculation. Int Angiol. 2008;27:81-85.
5. Les Laboratoires Servier. Daflon 500: summary of product characteristics. http://www.servier.com/sites/default/ files/Daflon500-spc.pdf. Accessed February 23, 2016.
6. Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413-420.
7. Katsenis K. Micronized purified flavonoid fraction (MPFF): a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr Vasc Pharmacol. 2005;3:1-9.
8. Silvestro L, Tarcomnicu I, Dulea C, et al. Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquidchromatography- mass spectrometry and ion mobility mass spectrometry. Anal Bioanal Chem. 2013;405:8295-8310.
9. Garner RC, Garner JV, Gregory S, Whattam M, Calam A, Leong D. Comparison of the absorption of micronized (MPFF at a dose of 500 mg) and nonmicronized 14C-diosmin tablets after oral administration to healthy volunteers by accelerator mass spectrometry and liquid scintillation counting. J Pharm Sci. 2002;91:32-40.
10. Cyrino FZ, Bottino DA, Lerond L, Bouskela E. Micronization enhances the protective effect of purified flavonoid fraction against postischaemic microvascular injury in the hamster cheek pouch. Clin Exp Pharmacol Physiol. 2004;31:159- 162.
11. Cospite M, Dominici A. Double blind study of the pharmacodynamic and clinical activities of 5682 SE in venous insufficiency. Advantages of the new micronized form. Int Angiol. 1989;8:61- 65.
12. Boutin JA, Meunier F, Lambert PH, et al. In vivo and in vitro glucuronidation of the flavonoid diosmetin in rats. Drug Metab Dispos. 1993;21:1157-1166.
13. Erlund I, Meririnne E, Alfthan G, Aro A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J Nutr. 2001;131:235-241.
14. Kanaze FI, Kokkalou E, Georgarakis M, Niopas I. A validated solid-phase extraction HPLC method for the simultaneous determination of the citrus flavanone aglycones hesperetin and naringenin in urine. J Pharm Biomed Anal. 2004;36:175-181.
15. Nielsen IL, Chee WS, Poulsen L, et al. Bioavailability is improved by enzymatic modification of the citrus flavonoid hesperidin in humans: a randomized, double-blind, crossover trial. J Nutr. 2006;136:404-408.
16. Barbe R, Amiel M. Pharmacodynamic properties and therapeutic efficacy of MPFF at a dose of 500 mg. Phlebology. 1992;7:41- 44.
17. Ibegbuna V, Nicolaides AN, Sowade O, Leon M, Geroulakos G. Venous elasticity after treatment with MPFF at a dose of 500 mg. Angiology. 1997;48:45-49.
18. Nicolaides A, Kakkos S, Eklof B, et al. Management of chronic venous disorders of the lower limbs – guidelines according to scientific evidence. Int Angiol. 2014;33:87-208.
19. Pascarella L. Daflon and the protection of venous valves. Phlebolymphology. 2016;23:20-30.
20. Takase S, Lerond L, Bergan JJ, Schmid- Schönbein GW. Enhancement of reperfusion injury by elevation of microvascular pressures. Am J Physiol Heart Circ Physiol. 2002;282:H1387-H1394.
21. Takase S, Delano FA, Lerond L, Bergan JJ, Schmid-Schönbein GW. Inflammation in chronic venous insufficiency: is the problem insurmountable? J Vasc Res. 1999;36:3-10.
22. Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28:484-493.
23. Pascarella L, Lulic D, Penn AH, et al. Mechanisms in experimental venous valve failure and their modification by MPFF at a dose of 500 mg. Eur J Vasc Endovasc Surg. 2008;35:102-110.
24. Tsoukanov YT, Tsoukanov AY, Nikolaychuk A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology. 2015;22:18-24.
25. Behar A, Lagrue G, Cohen-Boulakia F, Baillet J. Capillary filtration in idiopathic cyclic edema–effects of MPFF at a dose of 500 mg. Nuklearmedizin. 1988;27:105-107.
26. Galley P, Thiollet M. A double-blind, placebo-controlled trial of a new venoactive flavonoid fraction (S 5682) in the treatment of symptomatic capillary fragility. Int Angiol. 1993;12:69-72.
27. Cotonat A, Cotonat J. Lymphagogue and pulsatile activities of MPFF at a dose of 500 mg on canine thoracic lymph duct. Int Angiol. 1989;8(suppl 4):15-18.
28. Benoit JN. Study of the effects of S 5682 (MPFF at a dose of 500 mg) on intestinal lymph flow and lymphatic pumping. Study Report. 1995.
29. Mc Hale NG, Hollywood MA. Control of lymphatic pumping: interest of MPFF at a dose of 500 mg. Phlebology. 1994;9(suppl 1): 23-25.
30. Amiel M, Barbe R, Revel D. Etude de la relation dose/effect de MPFF at a dose of 500 mg par pléthysmographie chez l’homme [in French]. J Int Med. 1987;88:19-21.
31. Damon M, Flandre O, Michel F, Perdrix L, Labrid C, Crastes de Paulet A. Effect of chronic treatment with a purified flavonoid fraction on inflammatory granuloma in the rat: study of prostaglandin E2 and F2 alpha and thromboxane B2 release and histological changes. Arzneimittelforschung. 1987;37:1149-1153.
32. Meyer O. Safety of use of MPFF at a dose of 500 mg confirmed by acquired experience and new research. Phlebology. 1992;7:64-68.
33. Guillot B, Guilhou JJ, de Champvallins M, Mallet C, Moccatti D, Pointel JP. A long term treatment with a venotropic drug: results on efficacy and safety of MPFF at a dose of 500 mg in chronic venous insufficiency. Int Angiol. 1989;8:67-71.
34. Raju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Ann Vasc Surg. 2007;21:790-795.
35. Kahn SR, Shapiro S, Wells PS, et al; SOX Trial Investigators. Compression stockings to prevent post-thrombotic syndrome: a randomized placebo-controlled trial. Lancet. 2014;383:880-888.
36. Rabe E, Guex JJ, Puskas A, Scuderi A, Fernandez Quesada F; VCP Coordinators. Epidemiology of chronic venous disorders in geographically diverse populations: results from the Vein Consult Program. Int Angiol. 2012;31:105-115.
37. Martinez MJ, Bonfill X, Moreno RM, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev. 2005:CD003229.
38. Rabe E, Agus GB, Roztocil K. Analysis of the effects of micronized purified flavonoid fraction versus placebo on symptoms and quality of life in patients suffering from chronic venous disease: from a prospective randomized trial. Int Angiol. 2015;34:428-436.
39. Allaert FA. Meta-analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31:310-315.
40. Saveljev VS, Pokrovsky AV, Kirienko AI, Bogachev VY, Zolotukhin IA, Sapelkin SV. Stripping of the great saphenous vein under micronized purified flavonoid fraction (MPFF) protection (results of the Russian multicenter controlled trial DEFANCE). Phlebolymphology. 2008;15:45-51.
41. Veverkova L, Jedlicka V, Wechsler J, Kalac J, et al. Analysis of the various procedures used in great saphenous vein surgery in the Czech Republic and benefit of MPFF at a dose of 500 mg to postoperative symptoms. Phlebolymphology. 2006;13:195-201.
42. Bogachev VY, Golovanova OV, Kuznetsov AN, Sheokyan AO; DECISION Investigators Group. Can micronized purified flavonoid fraction (MPFF) improve outcomes of lower extremity varicose vein endovenous treatment? First results from the DECISION study. Phlebolymphology. 2013;20:181-187.
43. Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30:198-208.
44. Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7:303-308.
45. Ramelet AA, Boisseau MR, Allegra C, et al. Veno-active drugs in the management of chronic venous disease. An international consensus statement: current medical position, prospective views and final resolution. Clin Hemorheol Microcirc. 2005;33:309-319.