Obliteration of Varicose Veins with Superheated Steam
René MILLERET
3 Rue Pasteur – 34120 Pézenas – France
INTRODUCTION
Endovenous heating techniques have been in clinical use since 1999: longterm results are now available for both radiofrequency and endovenous laser.1 These results compare favorably with the gold standard: high ligation + stripping of the vein. Unfortunately, the cost of the equipment and disposable items necessary to implement these methods is still too high for many health systems in the world. The ideal technique would combine the advantages of currently used methods, and obviate their drawbacks. Heating the vein with superheated steam may provide significant medical and economic advantages.
PRINCIPLE
Water can be found in three physical states: ice, liquid, and vapor. In ice, H2O molecules stick to each other. If heat is added, they become separated by less than 1 molecular diameter: this is the liquid phase, water as we drink it. If more heat is added, molecular agitation increases and H2O molecules leave the surface as water vapor. This process is reversible: when vapor cools down as liquid, it gives back to surrounding molecules the heat which was used to change its state. A lot of heat is necessary to achieve the transition, thus vapor contains a lot of “latent heat” which can be used for heating tissues. As an example, more than 2000 KJ are needed to vaporize one liter of water at atmospheric pressure. Transition from liquid to vapor occurs at a temperature which is a function of pressure in the liquid: we all know that at atmospheric pressure this temperature is 100° C. If the pressure is increased to several hundred times atmospheric pressure, the steam will be emitted at a temperature up to several hundred degrees Celsius: it is called “superheated steam”.
EQUIPMENT
The equipment was developped by Cerma Vein (Archamps – France). To emit steam at 150°C, we pressurize water, then force it through a very small diameter tube (0.1 mm) heated by an electrical current. The tube is at the tip of a handpiece (Figure 1) to which a catheter can be connected. The single-use catheter is a thin tube of stainless steel coated with Teflon. At the tip of the catheter, steam is emitted at 120°C. The 1.2 mm (5F) catheter emits vapor near the tip through 2 lateral holes (Figure 2). A generator console controls the whole process and pressurizes the water.
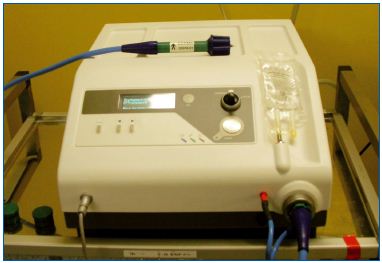
Figure 1. The generator with the handpiece
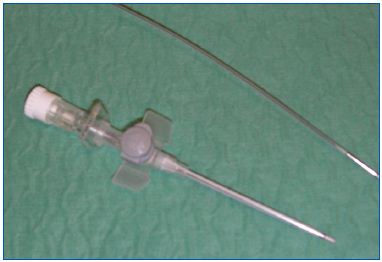
Figure 2. The endovenous catheter
DEVELOPMENT PHASE
We have conducted studies on freshly stripped segments of great saphenous veins (GSVs). This allowed us to select the best way to deliver steam in order to obtain shrinking of the collagen in the vein wall. Best results were obtained with 2 pulses of 45 J/cm per centimeter of vein–which is close to what is delivered by endovenous lasers. Macroscopically, immediate diameter reduction of the GSV was seen (Figure 3), with separation of the endothelial layer and media. No damage was seen in the adventitia and no perforation was evidenced. Microscopically, these findings were confirmed by widening of the media, which proved the lesion of collagen fibers (Figure 4).
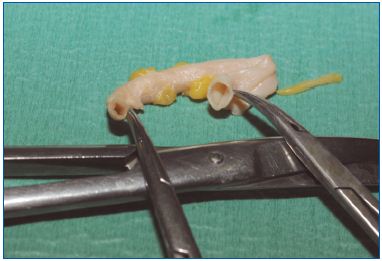
Figure 3. Macroscopic view of heated vein on the left , untreated
on the right
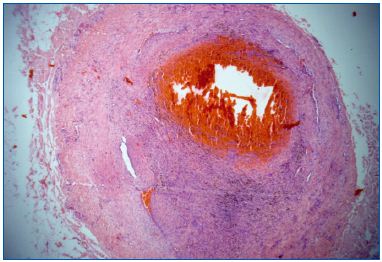
Figure 4. Microscopic view of animal control
Six ewes were used for animal studies. The external saphenous vein of the posterior limb was treated. Ten limbs were heated by steam, and 2 by radiofrequency (Closure® catheter) for comparative purposes. Different heating profiles were tested, either with or without tumescence around the vessel. Temperatures were measured peri-operatively at different levels: skin, directly around the vein by thermocouple, and in the inferior vena cava. Blood temperature was unchanged in this collecting trunk. Peri-venous temperature was elevated to 45°C without tumescence, and 37°C with tumescence. Skin temperature was not modified if tumescence was applied.
No hemolysis was observed in blood samples. General parameters were not affected, except in 1 sheep which had tachycardia, but independently of heating: it was due to an anesthetic problem.
The veins were harvested at 1 month (4 limbs) and 3 months (8 limbs). No general complication was observed. There was no infection and no necrosis or inflammatory reaction of the peri-venous tissues. Veins heated at less than 45 J/cm were closed in some segments and remained open in others. Veins heated at 90 J/cm were closed along their full length.
Microscopic studies showed disappearance of the endothelial layer and the presence of fibrotic tissue in the lumen extending into the media, but no damage to the adventitia.
APPLICATION TO SURGICAL TECHNIQUE
The treatment protocol follows the same steps as other thermal obliteration techniques. A duplex probe in a sterile cover is prepared. Sterile gel is not necessary, as we had good results by using saline or anesthetic solution applied with a soaked gaze.
Vein entrance:
Phlebotomy may be performed, but a transcutaneous approach is preferred. If the vein is of small caliber (often due to stress or cold-induced spasm), a tourniquet can be applied upward on the limb to increase pressure. The examination table can also be tilted to the anti- Trendelenburg position. A small amount of local anesthesia is injected with a fine (30G) needle at the point of puncture. To enter the vein, 2 devices can be used: a 16G infusion catheter or a 5F Seldinger puncture set. The latter is more expensive, but for small veins the smaller caliber of the needle can make it easier to obtain access. And a non-reflux valve is provided, avoiding oozing of blood during the procedure.
Transversal or longitudinal imaging of the vein can be used while puncturing the skin, and it is often useful to go from one to the other during the procedure. If several unsuccessful attempts are made, it is best to try upward at another location.
Catheterization:
Before entering the catheter in the vein, we measure on the skin the distance from the junction and apply a sterile tape at the level where we might stop (Figure 5). The catheter is pushed in the vein through the infusor or introducer. Upward progression should be easy and smooth, holding it between thumb and index without exerting any pressure. If resistance is encountered and progression stops, the duplex probe is used to image the tip without trying to go further by force and to check why it is blocked. It is usually at the level of a valve or of a localized dilatation. To get through, the catheter is withdrawn for 2/3 centimeters and pushed up while compressing the vein with the other hand or with the probe. Continued blockage is exceptional if the vein has not been previously sclerosed or thrombosed. If, nonetheless, there is still a blockage, 2 solutions can be applied: either re-enter just above the block, or if an F5 Seldinger introducer was used, try to go through with a guidewire, then slide in an F5 straight angiographic catheter and, after removing the guidewire, slide the steam catheter in it. Even though catheterization is easy, it is mandatory to use duplex ultrasound to check that the whole length of the catheter is in the vein lumen (
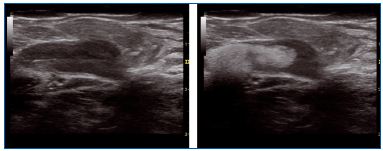
Figure 5. Steam in a dilated junction
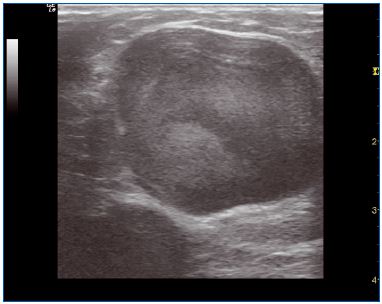
Figure 6. Large trunk before treatment
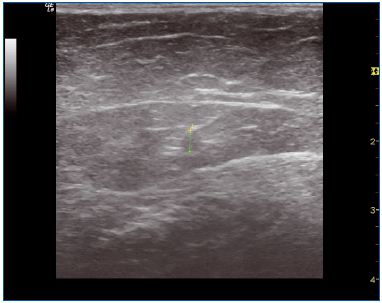
Figure 7. Large trunk 1 year after treatment
Checking the junction:
The safe distance to the junction is 3 cm. Longitudinal duplex scan is used to measure it. If there is an aneurysmal dilatation (anterior dilatation by jet effect) (Figure 8), the tip can be drawn nearer, but when heating the junction must be compressed with the probe applied transversally to avoid heating in the femoral vein.
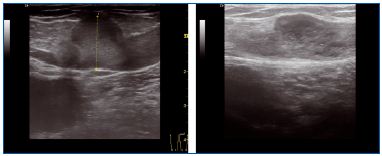
Figure 8. Aneurysmal dilatation before and immediately after
treatment
Ultrasound-guided local anesthesia:
Compression of the vein is advisable. We use ultrasoundguided local anesthesia: injection of small quantities of anesthetic solution around the vein using ultrasound guidance. Volumes are smaller than tumescent anesthesia, in the order of 150/200 cc for a GSV. We use a 1.4% bicarbonate solution to which is added lidocaine. Our proportions are 8 cc of 1% lidocaine for 90 cc of bicarbonate. The solution is applied with a 21G, 50 mm needle. The standard Klein solution can also be used, but more time is needed for the anesthesia to take effect. A peristaltic pump is helpful as it saves time.
Heating of the vein:
The first two pulses are not pure steam because the catheter is cold, so the steam emitted by the hand piece condenses before reaching the catheter tip. So when beginning heating at the junction, 5 pulses are sent: 2 nonheating + 3 heating. Then the catheter is withdrawn centimeter by centimeter. 2 pulses/cm for small veins: up to 7 mm (standing position), 3 pulses/cm in larger veins. If a localized dilatation has been mapped, or if the vein is more than 1.2 cm in diameter, >4 pulses are applied. As heat can accumulate inside the vein, it is best to stop heating for 5/10 seconds every 10 pulses.
Care must be taken to avoid skin burning at the entry point when removing the catheter. A specific marking is provided in the last 10 cm: when it appears, the entry device is removed with the heating catheter. At the last 2 centimeters, heating is stopped, a 5-second cooling time is allowed, and the catheter is extracted. A particular case is lipodermatosclerosis: it can be difficult to inject local anesthetic. We have successfully used skin cooling with a pad of gel to obtain anesthesia during heating.
Peri-operative check:
A correctly treated vein exhibits a thickened wall and is retracted: its caliber is 40 to 60% that measured preoperatively. Blood flow can sometimes still be observed in the lumen after distal compression in the first minutes after treatment, but will cease after 5/10 minutes when the thrombus is forming. If these modifications are not seen when checking the vein before removing the catheter, push it up again and repeat the procedure.
Post-operative care:
Compression stockings are recommended for 1 week, 2 weeks if the patient is standing at work. They are worn during the first day and first night, then only during the day. Pain is minimal or non-existent in most patients. If the patient complains of significant discomfort, we prescribe ibuprofen 400 mg: 1 tablet in the morning and 1 tablet in the evening for 5 days. Phlebectomy or foam sclerosis, if performed in the same session, is usually responsible for post-procedure pain.
Walking is resumed immediately. We advise the patient to wait until the next day before driving. As in other thermal techniques, prevention of deep vein thrombosis (DVT) and pulmonary embolism is advisable, using lowmolecular- weight heparin for 8 days in all patients, and up to 15 days when there is a personal or family history of thrombosis.
Post-operative checks:
If possible, a duplex check the next day is useful in detecting any complications— extension of a thrombus in the femoral vein, phlebitis of a tributary, DVT—and in confirming that the treated vein is closed. A second check is usually programmed at 4 weeks, especially if tributaries have been left to be treated after closure of the trunk.
The procedure is quite similar for small saphenous veins (SSVs). Vein entry is usually at the level of the mid-calf perforator. If this perforator is insufficient, entry should be proximal to it, so the obliteration of the saphenous trunk will suppress the reflux in the perforator in most patients (Figure 9).
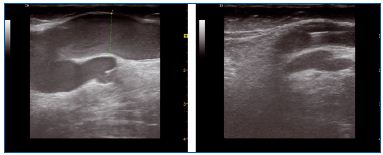
Figure 9. Small saphenous vein before and after treatment
If there is a bend of the vein toward the junction, the catheter can be advanced up to the bend without trying to go further down, as perforation of the vein wall could occur. If there is an insufficient axial posterior thigh vein or if the SSV has no junction at the popliteal level, the catheter can be pushed further with ultrasound guidance. Catheterization of a Giacomini communicating vein is usually achieved easily from below.
Heating parameters are similar to those used for a GSV: 2 pulses/cm for trunks under 7 mm and 3 pulses for larger trunks and localized dilatations. Local anesthesia is useful even if the patient is under general or spinal anesthesia because the nerve can be very close to the vein at the lower calf. If there is a common trunk with the gastrocnemius vein and this is to be spared, we advance the catheter up to 2 cm from the junction of both trunks and apply the probe transversally at the level of the junction to stop steam going further up.
PILOT CLINICAL STUDIES
We performed a first pilot clinical study on 10 limbs in 8 patients in 2007. All patients underwent duplex ultrasound 3 months, 1 year, and 2 years after treatment.
Material:
Female patients, aged between 36 and 65 years. Veins to be heated: 9 GSVs and 1 SSV.
Results:
No general complication was observed, in particular no DVT. Post-operative pain was minimal, so that no drugs were needed post-operatively. Return to activity was fast: all patients left the hospital on the day of intervention. One peri-operative complication occurred: a leg skin burn due to insufficient heat insulation of the catheter which came into contact with unprotected skin.
At the 24-month duplex ultrasound check, 8 of 10 treated veins were obliterated. In the other 2 veins there was a short length where there was repermeation between two tributaries at the lower part of the thigh. There were no symptoms.
Renate Van Den Bos and her co-workers from Professor Martino Neumann’s team at the Erasmus Medical Center treated 20 limbs in 19 patients.2 All were ambulatory and operated under tumescent local anesthesia. No complication was observed, incidents were limited to ecchymosis in 9 patients and one inflammatory reaction of the untreated vein under the point of puncture. Pain was minimal: 1 cm on a 10-cm visual analogue scale (VAS).
At the 6-month follow-up, venous scores had improved significantly as follows:
• Venous Clinical Severity Score (VCSS): from 5 to 2.5
• Aberdeen Varicose Vein Questionnaire (AVVQ): from 12.6 to 9.8
Thirteen limbs were totally occluded at 6 months, 5 had a partial reopening without reflux and less than 10 cm long, and 2 a partial reopening with reflux. But these last seven limbs had been treated with a lower energy per cm: one pulse/cm, 60 J versus two pulses/cm for the totally occluded cases.
Patient satisfaction score was 9.25 on a scale of 10.
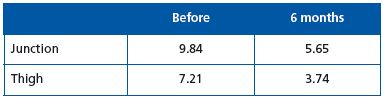
Eighty patients were treated in 4 centers: University Hospital of Besançon (Prof G. Camelot), private clinics in Lyon (Dr P. Nicolini), Montpellier (Dr R. Milleret) and Nancy (Dr D. Creton). Mean length treated was 42 cm for a mean diameter of 8 mm at mid-thigh. The only complication observed was a 1-cm extension of thrombus in the femoral vein in one of the first cases: it subsided without complications after low-molecular-weight heparin treatment.3
Pain was rated at 0.75 on 10 cm on the VAS at 8 days.
Vein resorption rate was better than 45% at 6 months:
Partial results at 1 year show an absence of reflux in 98.5% of 75 patients.
The SF-12 quality of life score was improved in both physical and mental terms: 49.99 versus 51.27 and 46.01 versus 52.05 at 6 months.
Saphenous trunks
We treated 164 saphenous trunks from 2007 to 2009 in our hospital: 117 GSVs and 47 SSVs. General anesthesia was used in 78 operations and local tumescent anesthesia in 76. No major complication was observed.
One patient had calf vein DVT which healed after lowmolecular- weight heparin treatment for 2 weeks. Three patients had minor skin burns at the entry point which healed in 4 to 6 weeks.
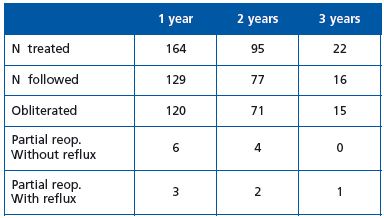
Follow-up results
We performed a comparative study of steam obliteration versus foam injection in large tributaries. The aim was to compare a chemical and a thermal technique in terms of efficiency, safety, cosmetic results, and patient satisfaction. Two groups of 20 patients were treated. Mapping was performed by the surgeon using ultrasound guidance. Foam was obtained using the Tessari method with 1% polidocanol for veins over 5 mm wide and 0.5% for smaller veins. No more than 10 cc was injected per session (Tegernsee Consensus).
Steam was applied using the generator and 18 g Venflon catheters for larger veins and 20G for smaller veins. Three to 6 pulses were applied at each entry point. Patients were followed up at 8/10 days, 1 month, and 6 months. A VAS was used to assess post-operative pain and overall satisfaction. Cosmetic results were classified from “very good” to “bad” in 4 steps.
All patients had a check-up at 8 days and 1 month. Three did not show at 6 months, 2 in the foam group and one in the steam group. No general complication was observed. One patient in the foam group had a transient visual disturbance. Inflammatory reactions were observed in 6 patients after foam injection and none after steam. Pain levels at 8 days were 2.4 after foam and 0.5 after steam.
At 1 month, blood extraction by a 16G needle under local anesthesia was performed in 12 patients of the foam group and 6 after steam obliteration. Pigmentation was present at 1 month in all these 12 patients and in 4 out of 6 in the steam group. It was still visible at 6 months in 4 patients after foam and 2 after steam.
Cosmetic results were assessed by the patient at 6 months: they were good or very good for 10 out of 18 after foam and for 15 out of 19 after steam. Our conclusion was that both techniques are safe. Steam leads to less pain and pigmentation due to the thickening of the vein wall, which implies a smaller lumen after treatment.
DISCUSSION:
Steam obliteration may have some advantages over current techniques. It is safe. The only by-product of this technique is water. As each pulse vaporizes only 0.08 cc of water, a GSV can be obliterated with 2 to 3 cc of water, a small quantity which will not induce hemolysis.
The mechanism of action is close to that of radiofrequency, with evenly distributed heat, as opposed to the more irregular pattern observed after endovenous laser with a bare fiber.4 Thus the endoluminal clot is minimal, and inflammatory reactions are rarely observed even in large trunks. The absence of perforation and the fast heat exchange with the vein wall lowers the risk of injury to surrounding structures. External cooling may be used when the vein is close to the skin, and obviate the need for tumescence. Further studies of this question are planned.
The catheter is thin and its flexibility is close to that of a guidewire: vein entrance is easier and can be achieved in most patients without Seldinger catheterization. Tortuosities can be passed by gently moving the vein under the skin using ultrasound guidance. As with any endothermal method, sufficient heating per centimeter is essential to obtain long-term obliteration.
Compared with foam obliteration, water vapor has several advantages. No chemical is used other than water, so there is no risk of anaphylactic shock. There is a reduced risk of DVT. It is not contraindicated if a persistent foramen ovale has been diagnosed. And, as shown in our study, pain due to inflammation and pigmentation are minimal. Lastly, the procedure is faster than phlebectomy and requires no incisions (a comparative study is under way).
CONCLUSIONS
These results show that superheated steam is efficient in obliterating superficial veins, which remain occluded after 2/3 years. Duplex imaging and resorption rates are very similar to those observed after Closure Fast® RF obliteration. The reduction in diameter is in line with what we found in a study of 25 limbs treated with Closure Plus® catheters.5 A randomized, multicenter study of water vapor versus endovenous laser is being performed at the Erasmus Medical Center (Rotterdam, Prof Martino Neumann) and 2 other Dutch centers to evaluate this new technique in a larger number of patients. The ability to obliterate tributaries with the same generator is promising as current heating techniques are mainly applied to saphenous trunks.

REFERENCES
1. Van Den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg. 2009;49:230-239.
2. Van Den Bos RR, Milleret R, Neumann M, Nijsten T. Proof-of-principle study of steam ablation as novel thermal therapy for saphenous varicose veins. J Vasc Surg. 2011;53:181-186.
3. Kabnick LS, Berland T. Heat induced thrombosis of the femoral vein after endovenous ablation for the great saphenous vein: clinical relevance? In: Wittens C, ed. Best Practice in Venous Procedures. Turin, Italy: Edizioni Minerva Medica; 2010:111-116.
4. Proebstle T. Sandhofer M , Kargl A. Thermal damage of the inner vein wall during endovenous laser treatment: key role of energy absorption by intravascular blood. Dermatol Surg. 2002;28:596-600.
5. Milleret R, Garandeau C, Brel D, Allaert FA. Sclérose des Grandes Veines Saphènes à la mousse: Alpha-Technique [in French]. Phlébologie. 2004;57:15-18.
