Advantages of early thrombus removal and how to perfect the technical and clinical outcomes
Houman Jalaie, MD, PhD
European Venous Center,
Department of Vascular Surgery,
University Hospital RWTH Aachen,
Germany
Natasha Hasemaki, MD
2nd Department of Vascular
Surgery, Laiko General Hospital,
University of Athens, Greece
Athanasios Katsargyris,
MD, PhD
2nd Department of Vascular
Surgery, Laiko General Hospital,
University of Athens, Greece
Chris Klonaris, MD, PhD
2nd Department of Vascular
Surgery, Laiko General Hospital,
University of Athens, Greece
Efthymios D. Avgerinos,
MD, FACS, FEBVS
2nd Department of Vascular
Surgery, Laiko General Hospital,
University of Athens, Greece
ABSTRACT
Deep venous thrombosis (DVT) is a source of morbidity by way of short term, disabling symptomatology and mid-long-term postthrombotic syndrome (PTS), particularly when involving the iliofemoral segment. Catheter-directed interventions for acute iliofemoral DVT have been increasingly used over the past decade to alleviate early symptoms and reduce the incidence and severity of PTS. To achieve these clinical benefits in a safe and durable way certain steps need to be applied involving patient selection, technique of thrombus removal, stenting (or not), and follow-up.
Introduction
Deep venous thrombosis (DVT) incidence keeps rising as our population is aging; the numbers of surgical procedures are growing; and our diagnostic strategies are improving.1 Whereas associated pulmonary embolism (PE) can infer significant morbidity and mortality, it is not as frequent; the main clinical burden of a DVT involves the short-term disabling symptomatology and a mid-long-term postthrombotic syndrome (PTS), particularly when involving the iliofemoral segment.2-4 Following an iliofemoral DVT, roughly 50% will develop PTS that can be a very debilitating condition with substantial health care costs.2-7
Catheter-directed interventions for acute iliofemoral DVT have been increasingly used over the past decade to alleviate early symptoms and reduce the incidence and severity of PTS.8-11 Given that these procedures are generally offered to a younger population, unless they are successful in the long term, no actual benefit can be claimed, putting patients at risk and raising health care costs. To achieve the clinical benefits of an intervention in a safe and durable way, certain steps need to be applied involving patient selection, technique of thrombus removal, stenting (or not), and follow-up.
Patient selection
The conflicting results of existing randomized trials have been criticized on the basis of diverse patient-inclusion criteria (eg, femoropopliteal DVTs combined with iliofemoral DVTs) and technical variations (eg, stenting rates, timing of intervention, inflow optimization, etc).11-18 There is little doubt though that these trials paved the way toward better patient selection to maximize benefits in terms of PTS severity reduction and quality of life (QOL) improvement, and this benefit can extend beyond 2 years after treatment.12-15 The lytic trials confirmed this benefit in good-risk symptomatic patients with iliofemoral DVT, provided intervention is done early enough, ideally within a 2-week window.11-15 Within this time window, thrombus is still noncollagenous (softer) and the valve function may be salvaged. Novel technologies have opened up this time window, but the actual benefit is yet to be seen. Based on these data, the European Society of Vascular Surgery and the International Union of Angiology in their most recent guidelines recommend early thrombus removal strategies in selected good-risk symptomatic patients with iliofemoral DVT.4,8 Whereas a femoropopliteal DVT can also lead to high PTS rates, based on the available data, an intervention should generally not Catheter-directed interventions for acute iliofemoral DVT have been increasingly used over the past decade to alleviate early symptoms and reduce the incidence and severity of PTS.8-11 Given that these procedures are generally offered to a younger population, unless they are successful in the long term, no actual benefit can be claimed, putting patients at risk and raising health care costs. To achieve the clinical benefits of an intervention in a safe and durable way, certain steps need to be applied involving patient selection, technique of thrombus removal, stenting (or not), and follow-up. be considered.4 Further work is needed, and an industry sponsored randomized trial is under way.19
In our practice, treatment of the threatened-limb population is not delayed, but the symptomatic ones are observed for a couple of days on anticoagulation and compression before any decision to intervene, as conservative treatment may sometimes allow complete symptom resolution.9,10,20 It is important to assess the symptoms on exertion, as at bedside patients rarely experience symptoms. For those whose symptoms persist, there is a good indication for intervention. Whereas the ideal timing is within 2-3 days, the traditional cut-off for intervention has been 2 weeks, but successful outcomes with newer-generation devices can also be achieved up to 1 month after the acute event.21 In an ATTRACT study (Acute venous Thrombosis: Thrombus Removal with Adjunctive Catheter-directed Thrombolysis) subanalysis, catheter intervention (vs anticoagulation alone) was beneficial with regard to PTS severity and QOL at 2 years for those who had higher clinical severity at baseline.22 So, for iliofemoral DVTs with no or mild symptoms an intervention cannot be clearly justified.
Technique
A sound technique that respects the principles of any deep venous intervention (inflow, outflow, conduit) is of paramount importance for a durable outcome. Suggestions for a required standard endovascular toolkit is summarized in Table I.
Before the decision to intervene, thrombus within the iliofemoral segment needs to be confirmed. Aside from a baseline duplex scan, a cross-sectional magnetic resonance (MR) venogram or computed tomography (CT) venogram (abdomen, pelvis, and upper leg) can facilitate operative planning by documenting the extent of the thrombus and uncovering unusual anatomies (eg, duplicated cava, inferior vena cava [IVC] aplasia, etc) or chronic venous obstruction. Decision-making and technique selection are summarized in Figure 1.
Inferior vena cava filter
A theoretical risk of a catheter intervention is iatrogenic PE related to the instrumentation of fresh thrombus.
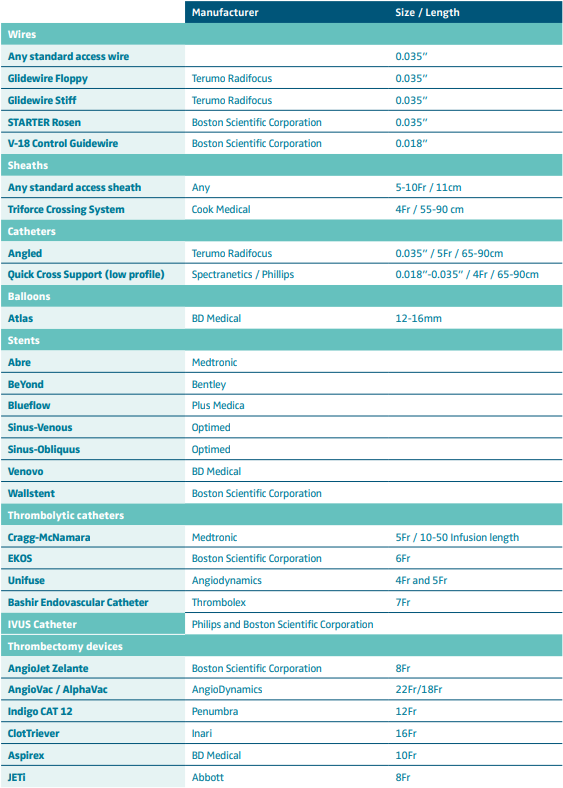
Table I. Suggested endovascular toolkit for the endovascular management of acute deep venous thrombosis. These are only a few suggested options based on the author’s experience and can cover the vast majority of cases.
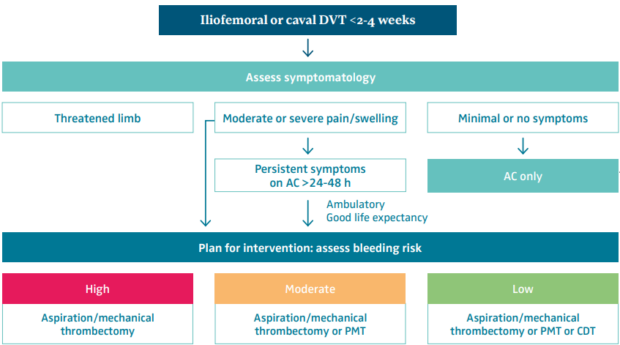
Figure 1. Patient and treatment selection for iliofemoral deep venous thrombosis. Abbreviations: AC, anticoagulation; CDT, catheter-directed thrombolysis; DVT, deep venous thrombosis; PMT, pharmacomechanical thrombectomy. Reprinted by permission from reference 9: Avgerinos and Jalaie. Phlebolymphology. 2023;30(3):118-124.
Although a small randomized trial has indicated a higher rate of clinically significant PE in patients not receiving an IVC filter, there was no mortality difference, and subsequent contemporary studies recommend highly selective IVC filtration. PE can be unavoidable, but they are rarely clinically meaningful for otherwise good-risk patients, and placement of an IVC filter may introduce complexity and other potential risks.23,24 In our experience, IVC filters are rarely used irrespective of the type of catheter intervention. Coexisting PE with significant thrombus burden and/or clinical instability (do these patients need DVT thrombectomy?) or mobile iliocaval thrombus can be acceptable indications.
Access
Treating an iliofemoral DVT will typically require low popliteal (or small saphenous) vein access with the patient in prone positioning. This will guarantee good control and imaging of the femoral bifurcation that is the gatekeeper of iliac vein patency. The presence of popliteal thrombus is not a contraindication to access the vein. Use of ultrasound and a micropuncture system is preferrable to minimize bleeding complications, particularly if thrombolytics are considered. If needed, the popliteal vein can accept large sheaths to accommodate the standard venous stent delivery systems (9 or 10 Fr) and even up to 16 Fr after serial dilatations for larger thrombectomy devices. Proximal tibial access can also be obtained and can accommodate 9- to 10-Fr sheaths. An ipsilateral mid-femoral puncture in the supine position can also be sufficient for isolated iliac or caval DVT.
A 5-Fr short sheath, a starter 0.035-inch wire, and a standard guiding catheter are typically enough to cross fresh thrombus and obtain images at the femoropopliteal and iliocaval segments. After establishing access and crossing the lesion, a baseline intravascular ultrasound (IVUS) can be performed to evaluate the extent of the thrombus burden, the chronicity, and the anatomy.
Thrombolysis versus thrombectomy
Contemporary practice offers multiple technical alternatives for thrombus removal, largely divided as thrombolytic and nonthrombolytic techniques, though many times a combination may be best. There is no ideal device or technique: aspiration thrombectomy can bail out a failed thrombolytic technique, and thrombolysis can bail out a failed aspiration thrombectomy. In principle though, thrombectomy techniques have recently evolved toward thrombolytic-free interventions, altering the safety profile and the complex hospital logistics (eg, need for an intensive care unit [ICU]). Contemporary practice has shifted toward a single-session mechanical intervention without ICU stay.
Thrombolytics, for patients at low bleeding risk, still remain relevant and should be considered in certain clinical scenarios, eg, establishing tibial inflow in an “ascending” thrombosis, IVC aplasia, extensive DVT to achieve a cleaner vein before embarking on mechanical thrombectomy, or if after debulking a large amount of thrombus with mechanical thrombectomy there is still significant residual thrombus.9,10,20 Thrombolysis can be performed using a multi-sidehole standard catheter or the EKOS catheter that incorporates ultrasound probes to accelerate fibrin separation. It is essential to accommodate the entire infusion catheter segment (available in up to 50 cm) within the clot; otherwise, the lytic agent will escape through the holes of least resistance into the blood stream. Contemporary thrombolysis protocols can vary in time between 6 to 12 hours, and dosage should typically range between 0.5 to 2 mg/hour. Patients will need to be transferred to the ICU for monitoring during the dripping and returned to the interventional suite for termination of the procedure.
Aspiration or mechanical thrombectomy (MT) can be performed with any of the novel available devices on the market (Figure 2) with which the practitioner feels comfortable. The ultimate target of a successful thrombus removal is >90% extraction and provides optimal inflow (through the femoral and deep femoral veins) to the iliac segment (Figure 3). This will minimize the risk of early re-thrombosis or later PTS.25-28
Following thrombectomy, IVUS can be used to determine the completeness of thrombectomy and the need to place a stent, its diameter, and its length. IVUS has been shown in multiple studies to be superior for accurate lesion identification compared with plain venography, and its use improves long-term patency.29
Venous stenting
Several dedicated venous stents are available. Sufficient stenting of persistent lesions (chronic obstruction, residual thrombus, external compression) after thrombus removal seems to be a critical component of a clinically successful procedure.

Figure 2. Contemporary mechanical thrombectomy devices: AngioJet (Boston Scientific Corporation), ClotTriever (Inari Medical), JETi (Abbott), Aspirex (BD Medical), Indigo CAT 12 (Penumbra), AngioVac (AngioDynamics). Reprinted by permission from reference 9: Avgerinos and Jalaie. Phlebolymphology. 2023;30(3):118-124.
Accumulating experience favors stenting, most likely exceeding 50% of intervened patients. The rationale is to treat the underlying DVT precipitating factor (typically an iliac vein compression—May Thurner lesion) or cover thrombotic collagenous material that creates a significant stenosis (>50% IVUS area reduction, frequently corresponding to stenosis >70% of the diameter).30-33 Note, however, that DVT itself causes inflammation, and along with the catheter manipulations, they can cause luminal stenosis that will eventually resolve, thus stents can be spared.34 IVUS and appropriate expertise in the interpretation of the images will better guide the need for stent placement.20
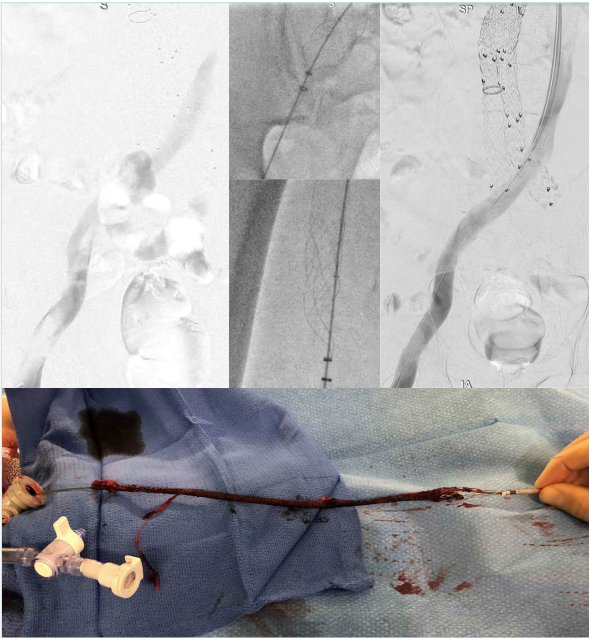
Figure 3. Iliofemoral deep venous thrombosis following endovascular aneurysm repair and heparin-induced thrombocytopenia. Mechanical thrombectomy using the ClotTriever device (Inari). No stent was required.
When a stent will be deployed it needs to provide an adequate, well-expanded conduit and ensure good inflow and outflow. Consequently, in many cases, it may be necessary to stent from the iliocaval confluence down to the common femoral vein. Care should be taken to prevent jailing of the contralateral common iliac vein as well as jailing of the deep femoral vein when extending distally. The common iliac vein is typically stented with a 14- to 16-mm stent, and the external iliac/ common femoral veins with a 12- to 14-mm stent. The length of the iliac stent should also be long enough (≥8 cm) to anchor at the external iliac segment, preventing migration and avoiding an acute angulated landing at the iliosacral curvature (Figure 4).35-39
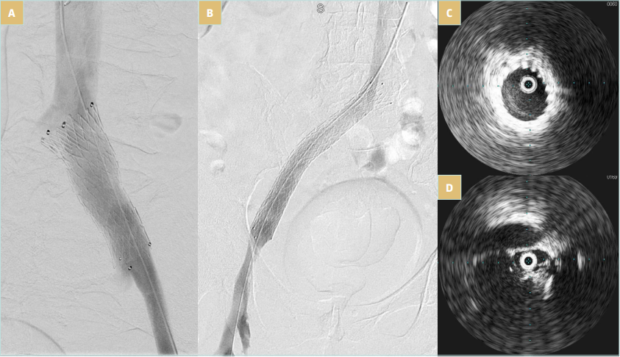
Figure 4. Venovo (BD Medical) stent placement after thrombectomy. A) Appropriate diameter of 16 mm, but short with a risk of migration; B) appropriate diameter of 16 mm and length of 120 mm; C) iliac vein compression after thrombectomy and before stenting as seen in intravascular ultrasound; D) well-expanded stent as seen in intravascular ultrasound.
Perioperative care and surveillance
The patient should remain on bed rest for 2 to 4 hours to allow for hemostasis; the index leg needs to be tightly wrapped; and after hospital discharge, thigh-high compression at 20 to 30 mm Hg should be encouraged for at least 1 month or until the swelling completely resolves. The patient should also be encouraged to drink plenty of fluids in order to minimize the effects of hemoglobinuria. Within 6 to 8 hours, the patient should be encouraged to ambulate.
Before discharge, a duplex ultrasound can identify early failures and need for reintervention. The patient should be discharged with a defined plan for anticoagulant therapy that is consistent with their risk of recurrence. For patients who received a stent, before initiating oral anticoagulation, low-molecular-weight heparin for 2 to 6 weeks is preferred owing to its anti-inflammatory effects. An antiplatelet agent for 6 months or indefinitely depending on the patient’s risk profile can be considered. Appropriate referral to hematology is warranted in patients with an unprovoked DVT or possible thrombophilia. A follow-up office visit is recommended at 2 to 4 weeks, at 3, 6, and 12 months, and annually thereafter with duplex ultrasound.40,41 Cross-sectional imaging can be needed on occasion in complex iliocaval reconstructions to evaluate patency.
Conclusions
Contemporary catheter interventions for symptomatic patients with acute iliofemoral DVT are generally safe and do not require prolonged hospitalization. As DVT rates are rising, awareness of these novel treatments and appropriate technical expertise within a multidisciplinary team can guarantee optimal results and ultimately a better QOL for our patients.

CORRESPONDING AUTHOR
Efthymios Avgerinos, MD, FACS, FEBVS
Athens Medical Center; Doctors’ Building,
2nd floor, Office Γ1, Kifisias 56 & Delfon,
151 25 Marousi, Athens, Greece
EMAIL: eavgerinos@vascularhealth.gr;
efavgerinos@gmail.com
References
1. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464-474.
2. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105-1112.
3. Broholm R, Sillesen H, Damsgaard MT, et al. Postthrombotic syndrome and quality of life in patients with iliofemoral venous thrombosis treated with catheter-directed thrombolysis. J Vasc Surg. 2011;54(6 Suppl):18S-25S.
4. Kakkos SK, Gohel M, Baekgaard N, et al. Editor’s Choice – European society for vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2020;61:9-82.
5. Magnuson EA, Chinnakondepalli K, Vilain K, et al. Cost-effectiveness of pharmacomechanical catheter directed thrombolysis versus standard anticoagulation in patients with proximal deep vein thrombosis: results from the ATTRACT trial. Circ Cardiovasc Qual Outcomes. 2019;12(10):e005659.
6. Babigumira JB, Black SA, Lubinga SJ, Pouncey AL. Cost effectiveness of early endovenous thrombus removal for acute iliofemoral deep vein thrombosis in the United Kingdom. Eur J Vasc Endovasc Surg. 2024;67(3):490-498.
7. Jalaie H, Avgerinos E. Early deep vein thrombosis intervention is cost effective and can only get better. Eur J Vasc Endovasc Surg. 2024;67(3):499.
8. Nicolaides AN, Fareed J, Spyropoulos AC, et al. Prevention and management of venous thromboembolism. International Consensus Statement. Guidelines according to scientific evidence. Int Angiol. 2024;43(1):1-222.
9. Avgerinos E, Jalaie H. Progress in the management of early thrombus removal. Phlebolymphology. 2023;30(3):118-124.
10. Avgerinos ED, Bouris V, Jalaie H. The emerging role of mechanical thrombectomy in acute DVT management. J Cardiovasc Surg (Torino). 2024;65(1):23-31.
11. Javed A, Machin M, Gwozdz AM, et al. Meta analysis of lytic catheter-based intervention for acute proximal deep vein thrombosis in the reduction of post-thrombotic syndrome. J Vasc Surg Venous Lymph Disord. 2023;11(4):866-875.
12. Haig Y, Enden T, Grøtta NEK, et al. Post thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label randomised controlled trial. Lancet Haematol. 2016;3:e64-e71.
13. Vedantham S, Goldhaber SZ, Julian JA, Kahn SR; for the ATTRACT trial. Pharmacomechanical catheter-directed thrombolysis for deep vein thrombosis. N Engl J Med. 2017;377(23):2240-2252.
14. Comerota AJ, Kearon C, Gu CS, et al. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis: analysis from a stratified multicenter randomised trial. Circulation. 2019;139(9):1162-1173.
15. Notten P, de Smet AAEA, Tick LW, et al. CAVA (ultrasound-accelerated catheter-directed thrombolysis on preventing post-thrombotic syndrome) trial: long-term follow-up results. J Am Heart Assoc. 2021;10:e018973.
16. Avgerinos EA, Chaer RA. The ATTRACTiveness of catheter-directed thrombolysis. J Vasc Surg Venous Lymphat Disord. 2018;6(3):303.
17. Nana P, Avgerinos E, Spanos K, Giannoukas A, Labropoulos N. Gaps arising from randomized controlled trials on thrombolysis for proximal deep vein thrombosis of the lower limb. J Vasc Surg Venous Lymphat Disord. 2022;10(1):196-199.
18. Go C, Chaer RA, Avgerinos ED. Catheter interventions for acute deep venous thrombosis: who, when and how. Vasc Endovasc Rev. 2020;3:e07.
19. Inari Medical begins DEFIANCE randomized clinical trial of ClotTriever system in DVT. Published January 12, 2023. Endovascular Today. https://evtoday.com/news/inari-medical-begins-defiance-randomized clinical-trial-of-clottriever-system-in-dvt
20. Avgerinos ED, Black S, van Rijn MJ, Jalaie H. The role and principles of stenting in acute iliofemoral venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2024;12(5):101868.
21. Abramowitz SD, Kado H, Schor J, et al; CLOUT Investigators. Six-month deep vein thrombosis outcomes by chronicity: analysis of the real-world ClotTriever Outcomes Registry. J Vasc Interv Radiol. 2023;34(5):879-887.e4.
22. Thukral S, Salter A, Lancia S, Kahn SR, Vedantham S. Predictors of clinical outcomes of pharmacomechanical catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: analysis of a multicenter randomized trial. J Vasc Interv Radiol. 2022;33:1161-1170.
23. Sharifi M, Bay C, Skrocki L. Role of IVC filters in endovenous therapy for deep venous thrombosis: The FILTER-PEVI (filter implantation to lower thromboembolic risk in percutaneous endovenous intervention) trial. Cardiovasc Intervent Radiol. 2012;35:1408- 1413.
24. Avgerinos ED, Hager ES, Jeyabaln G, Marone L, et al. Inferior vena cava filter placement during thrombolysis for acute iliofemoral deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2014;2:274-281.
25. Aziz F, Comerota AJ. Quantity of residual thrombus after successful catheter directed thrombolysis for iliofemoral deep venous thrombosis correlates with recurrence. Eur J Vasc Endovasc Surg. 2012;44(2):210-213.
26. Comerota AJ, Grewal N, Martinez JT, et al. Postthrombotic morbidity correlates with residual thrombus following catheter directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55(3):768-773.
27. Avgerinos E, Saadeddin Z, Abou Ali AN, et al. Outcomes and predictors of failure of iliac vein stenting after catheter directed thrombolysis for acute iliofemoral thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7(2):153-161.
28. Pouncey AL, Kahn T, Morris RI, Saha P, Thulasidasan N, Black SA. Risk factors and classification of reintervention following deep venous stenting for acute iliofemoral deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2022;10:1051-1058.
29. Tran LM, Go C, Zaghloul M, et al. Intravascular ultrasound evaluation during iliofemoral venous stenting is associated with improved midterm patency outcomes. J Vasc Surg Venous Lymphat Disord. 2022;10(6):1294-1303.
30. Meissner MH, Gloviczki P, Comerota AJ, et al. Early thrombus removal strategies for acute deep venous thrombosis: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2012;55:1449-1462.
31. Mahnken A, Thomson K, de Haan M, O’Sullivan G. CIRSE Standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37(4):889-897.
32. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006;44(1):136-143.
33. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48-56.
34. Jayaraj A, Thaggard, Lucas M. Technique of stent sizing in patients with symptomatic chronic iliofemoral obstruction – the case for intravascular ultrasound-determined inflow channel stenting and associated long-term outcomes. J Vasc Surg Venous Lymphat Disord. 2023;11(3):634-641.
35. Raju S, Buck WJ, Crim W, Jayaraj A. Optimal sizing of iliac vein stents. Phlebology. 2018;33(7):451-457.
36. Sayed MH, Salem M, Desai KR, O’Sullivan GJ, Black SA. A review of the incidence, outcome, and management of venous stent migration. J Vasc Surg Venous Lymphat Disord. 2022;10:482-490.
37. Robertson B, Shapiro J, Muck A, et al. Venous stent patency is independent of total stented length in nonthrombotic iliac vein and post-thrombotic venous stenoses. J Vasc Surg Venous Lymphat Disord. 2023;11:339-345.
38. Murphy EH, Johns B, Varney E, Buck W, Jayaraj A, Raju S. Deep venous thrombosis associated with caval extension of iliac stents. J Vasc Surg Venous Lymphat Disord. 2017;5:8-17.
39. Cheng CP, Dua A, Suh GY, Shah RP, Black SA. The biomechanical impact of hip movement on iliofemoral venous anatomy and stenting for deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8(6):953-960.
40. Sebastian T, Barco S, Engelberger RP, et al. Duplex ultrasound investigation for the detection of obstructed iliocaval venous stents. Eur J Vasc Endovasc Surg. 2020;60:443-450.
41. Avgerinos ED, Labropoulos N. Duplex criteria for iliocaval stent obstruction: sounds of a cry for validated data. Eur J Vasc Endovasc Surg. 2020;60(3):451.
