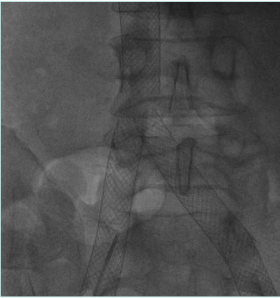
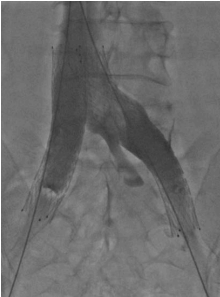
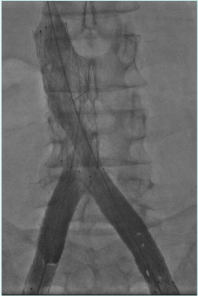
Treatment of vena cava obstruction and occlusion
Marzia Lugli, MD
Department of Cardiovascular
Surgery, Hesperia Hospital,
Modena, Italy
ABSTRACT
Obstruction of the inferior vena cava (IVC) is a significant contributor to chronic venous insufficiency (CVI). Patients may present with a wide range of symptoms, from being asymptomatic to suffering from severely debilitating conditions.
The causes of IVC obstruction can be congenital, related to malignancies, or nonmalignant. Among these, nonmalignant causes are the most common.
The protocol for selecting patients for cava recanalization and stenting must be strict. The clinical basis should guide the procedural approach for inferior cava obstruction.
The endovascular technique for addressing proximal deep vein obstruction is now well standardized. Special care should be taken to reconstruct the confluence when applied in the IVC.
Considering the low complication rates, satisfactory patency rates, and symptom improvement, the endovascular approach is worth considering to enhance the quality of life for these patients. Although additional high quality evidence is needed, the benefits outweigh the drawbacks for cava recanalization in selected individuals.
Introduction
Inferior vena cava (IVC) obstruction, associated or not with iliofemoral obstruction, is an important cause of chronic venous insufficiency (CVI). The clinical presentation may vary from asymptomatic patients to those with severe debilitating disease, leading to a dramatic compromise in the quality of life. There are several possible causes, primarily thrombotic events, neoplastic compression, surgical or traumatic interruptions, and congenital defects. Regardless of the cause of the obstruction, conservative treatments, Figure 1. Extensive acute iliocaval thrombosis. Clots are clearly visible inside iliac veins and the inferior vena cava. Figure 2. Cava bypass for right renal tumor vein wall infiltration. The cava has been harvested, and a bovine pericardium bypass has been constructed from the left renal vein down to the confluence of the iliac veins. such as anticoagulation and compression therapy, have long been the only therapeutic options due to the significant complication rates associated with open surgery for correcting cava obstruction. Nowadays, the endovascular technique for IVC obstruction management is the first-line procedural approach. There is undoubtedly a widespread and increasing tendency to treat cava obstruction utilizing recanalization/stenting, supported by good clinical results and a low complication rate.1
Inferior cava obstruction
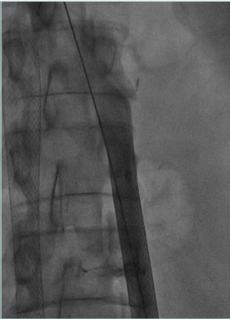
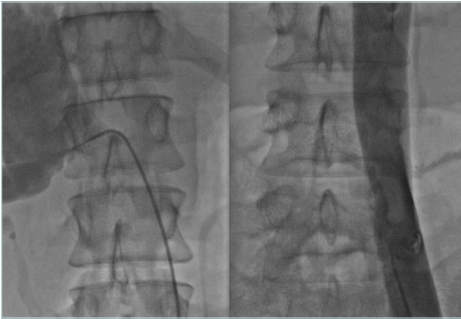
The IVC obstruction etiology can be congenital, malignant related, or nonmalignant. The nonmalignant etiology is the most frequent one, due to thrombotic events involving the cava and frequently iliac/femoral segments (Figure 1).2 Frequently, these events occur in young patients (eg, thrombosis caused by coagulation defects or trauma), leading to severe symptoms and signs such as disabling leg edema, venous claudication, and ulcers, as well as the whole spectrum of CVI manifestations. Even if cava obstruction may be well compensated at the beginning, usually over time the quality of life for these patients deteriorates progressively. In malignant-related obstruction, cava compression usually needs to be managed, even in compassionate situations for patients with a short life expectancy. Open surgical cava replacement may, in some cases, be considered when the vein wall is involved in tumoral infiltration (Figure 2). Cava congenital anomalies are quite rare. Cava duplication (Figure 3), as the result of abnormal persistence of the left supracardinal vein, and the left-sided cava (Figure 4) are not so infrequent and can be recanalized if needed. The cava interruption, due mainly to the failure of the right subcardinal vein to anastomose with the vitelline vein, is on the other hand difficult to manage given the absence of axiality (Figure 5).3
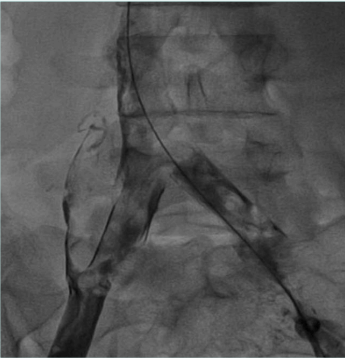
Figure 1. Extensive acute iliocaval thrombosis. Clots are clearly visible inside iliac veins and the inferior vena cava.
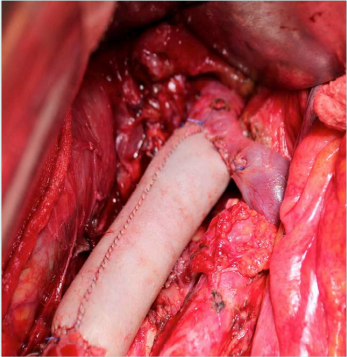
Figure 2. Cava bypass for right renal tumor vein wall infiltration. The cava has been harvested, and a bovine pericardium bypass has been constructed from the left renal vein down to the confluence of the iliac veins.
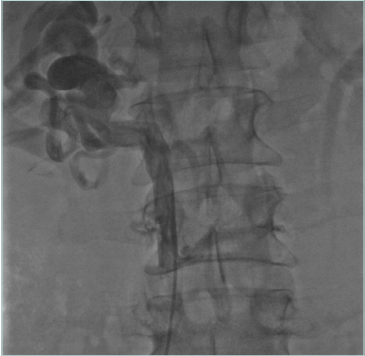
Figure 5. Congenital cava interruption. The cava (postthrombotic damage inside the vein) is stopped at the confluence of suprahepatic veins, visible on the left. There is no continuity with the right atrium.
Patient selection
The investigation protocol for selecting patients for cava recanalization/stenting should be rigorous. The procedural approach to inferior cava obstruction should be indicated on a clinical basis. The detection of a stenotic/occlusive image at diagnostic investigation does not represent an indication for treatment as underlined in the recent European Guidelines. In fact, the “Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs” of the European Society for Vascular Surgery (ESVS) has given a recommendation IIa, level B in this direction.4 In patients affected by CVI or by disabling venous claudication, the history of a previous episode of acute thrombosis suggests the screening for proximal obstruction. Ultrasound (US) investigation is the first-line examination to be performed; in the larger part of cases, US provides information about postthrombotic damage in the inferior cava system, flow spectrum alteration (ie, breath nonphasic flow), and rough cava alterations. Whenever a procedural approach is advisable, computed tomography venography (CTV), magnetic resonance venography (MRV), or phlebography are mandatory. Intravascular US (IVUS) is the gold standard for vein obstruction detection; so during the recanalization, its application is strongly recommended.5
Contraindications to cava recanalization are represented by the presence of uncorrectable coagulation alterations, as well as the impossibility to apply anticoagulation therapy. Moreover, the decision-making process should take into account the likelihood of a low benefit with intervention: patients with ineffective muscular pump function or patients that are nonambulatory typically do not improve significantly after recanalization. To this end, the preoperative plethysmographic assessment of the ejection fraction (EF) is an essential parameter, given that it can provide information about the calf pump function.6
Cava recanalization: the procedure
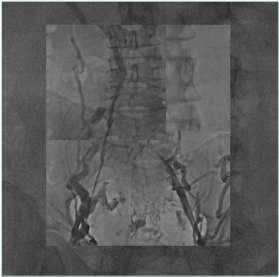
In cava obstruction recanalization, a multiple vein access procedure is usually required. There are several options for the access site (femoral vein, popliteal vein, internal jugular vein); the choice is led by the extent and localization of the steno-obstructive lesions to be treated, considering that cava postthrombotic obstruction is usually associated with iliac and common femoral damage (Figure 6). The most frequent choice is bilateral femoral half-thigh access combined with jugular access. Ultrasound-assisted catheterization is mandatory to avoid or reduce complications.
Venous angioplasty or stenting is a painful procedure. In the event of complex iliocaval obstruction, general anesthesia should be considered, both for pain occurrence and the possible long duration of the procedure.7,8
Once the sheath has been placed, a multiplanar venography is performed to assess the extent and characteristics of the lesions. It is important to understand if the cava is occluded or stenotic: this will make a strong difference in the aggressiveness of the procedure (eg, in occluded cases, it is a stronger approach) and will affect outcomes. Systemic anticoagulation with 100 units/kg of unfractionated heparin is undertaken and the activated clotting time during the procedure is monitored.
The obstructive lesions are crossed by means of different types of wires and supporting catheters; telescopic techniques and specific devices for crossing occlusions are usually considered. Predilatation is performed with progressive large-diameter balloons.
The diagnostic assessment before stenting should be carried out by IVUS. The IVUS probe is directed over the atrial confluence of the IVC, and the scanning is recorded with a pullback technique. A precise assessment of residual stenosis, lesion extent, and stent size requirement, and a decision about landing zones are undertaken.
The stent choice, from among those available on the market, depends on the specific characteristics of the clinical situation. The most used stent sizes are 20 to 24 mm for the IVC, 14 to 16 mm for the iliac veins, and 12 to 14 mm for extension into the common femoral vein (CFV). Balloon postdilation is recommended.
How to reconstruct the cava confluence via stenting is an essential point. Different strategies may be applied, depending on local anatomy, inflow issues, and device availability. The main problem is how the iliac stents should converge into the cava stent. It is possible to infold them into the cava stent, thus requiring appropriate sizing; a carina Y-stent configuration can be used with the drawback of residual gaps. A double-barrel stent can also be used, which may behave as a flow diverter. There is no data to help select the best configuration. Thus, the choice mainly depends on each individual case.9-11 (Figures 7 to 11)
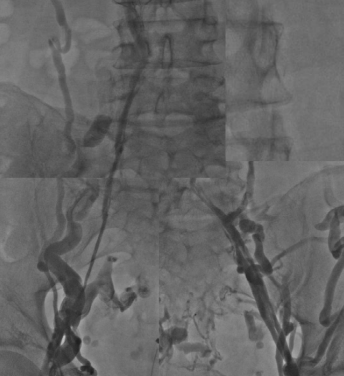
Figure 6. Extensive cava and iliofemoral postthrombotic obstruction. A cava filter is placed in the inferior vena cava.
A final check by IVUS and a completion venography are performed. Possible residual stenosis, adequate collapse of the collateral pathways, and adequate inflow are essential parameters to be assessed as predictors for patency.
At the end of the procedure, the venous access sheaths are removed, and an eccentric compression is performed. Elastic stockings are placed on, and intermittent pneumatic compression is applied until active ambulation.
Full anticoagulation is carried out; the anticoagulant agent and duration depend on the patient’s characteristics (ie, presence of an anticoagulation defect, the persistent risk of deep venous thrombosis).
Complications
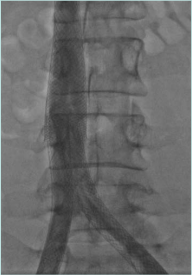
Recanalization/stenting of venous obstruction is to be considered a safe procedure. Pulmonary embolism rate, bleeding from access points, stent migration, and infections are negligible. Stent restenosis and recurrent thrombosis are the essential issues to be considered and monitored attentively during follow-up in order to provide adjunctive maneuvers (Figure 12).12
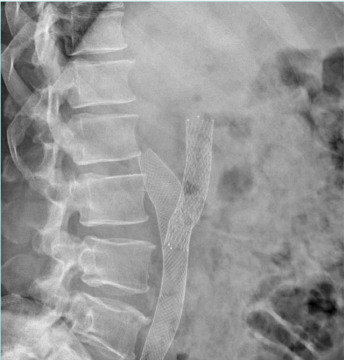
Figure 12. Proximal stent realignment for segmental occlusion. The cranial part of the original stent was occluded and a second one has been placed by means of fenestration.
Results
Analyzing results from deep venous obstruction recanalization reveals several notable issues. Firstly, there is a lack of uniformity in how the treated population is described, scored, and classified. Additionally, the definitions for measuring outcomes require improvement. Furthermore, the duration of follow-up remains relatively brief. Nevertheless, the complication rate is low, and the technical success is relatively high.
Regarding stent patency, primary obstruction presents better results compared with postthrombotic syndrome patients in all available meta-analyses. In a review by Williams and Dillavou,12 the 1-year primary patency rate for iliocaval cases is 79%. Recently, Morris et al reported primary patency rates of 75% (38% to 98%) and secondary patency rates of 91.5% (77% to 100%).13 Clinical outcomes are variable due to the heterogenicity of the population, ranging from a 64% to 82% improvement. It is important to highlight that iliocaval recanalization is cost-effective compared with conservative therapy alone.14
Conclusions
Inferior cava obstruction is a cause of severe CVI. Given the low rate of complications, satisfying patency rates, and improved symptoms, the endovascular approach should be considered to improve the quality of life in these patients. Even if more high-quality evidence is required, the ratio of pros and cons favors cava recanalization in selected patients.

CORRESPONDING AUTHOR
Dr Marzia Lugli,
Department of Cardiovascular Surgery,
Hesperia Hospital, Via Arquà 80/A,
41125 – Modena, Italy
EMAIL:
lugli@chirurgiavascoalremodena.com
References
1. de Graaf R, Estler A, Grözinger G. Inferior and superior vena cava reconstruction. Cardiovasc Intervent Radiol. 2024 Sep 24. doi: 10.1007/s00270-024-03867-x.
2. Erben Y, Bjarnason H, Oladottir GL, McBane RD, Gloviczki P. Endovascular recanalization for nonmalignant obstruction of the inferior vena cava. J Vasc Surg Venous Lymphat Disord. 2018;6(2):173-182.
3. Kim H, Labropoulos N, Blake AM, Desai K. Prevalence of inferior vena cava anomalies and their significance and impact in clinical practice. Eur J Vasc Endovasc Surg. 2022;64(4):388-394.
4. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice – European Society for Vascular Surgery (ESVS) 2022 Clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63(2):184-267.
5. Gagne PJ, Gasparis A, Black S, et al. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6(1):48-56.
6. Maleti O, Nicolaides A, Guerzoni S, Bergamo G, Lugli M. Residual volume fraction during walking using wireless air plethysmography in patients with chronic deep venous disease. J Vasc Surg Venous Lymphat Disord. 2022;10(2):423-429.
7. Raju S, Hollis K, Neglen P. Obstructive lesions of the inferior vena cava: clinical features and endovenous treatment. J Vasc Surg. 2006;44:820-827.
8. Murphy EH, Johns B, Varney E, Raju S. Endovascular management of chronic total occlusions of the inferior vena cava and iliac veins. J Vasc Surg Venous Lymphat Disord. 2017;5(1):47-59.
9. de Graaf R, de Wolf M, Sailer AM, van Laanen J, Wittens C, Jalaie H. Iliocaval confluence stenting for chronic venous obstructions. Cardiovasc Intervent Radiol. 2015;38:1198-1204.
10. Barbati ME, Gombert A, Toonder IM, et al. Iliocaval skip stent reconstruction technique for chronic bilateral iliocaval venous occlusion. J Vasc Interv Radiol. 2020;31:2060-2065.
11. Crowner J, Marston W, Almeida J, McLafferty R, Passman M. Classification of anatomic involvement of the iliocaval venous outflow tract and its relationship to outcomes after iliocaval stenting. J Vasc Surg Venous Lymphat Disord. 2014;2: 241-245.
12. Williams ZF, Dillavou ED. A systematic review of venous stents for iliac and vena caval occlusive disease. J Vasc Surg Venous Lymphat Disord. 2020;8(1):145-153.
13. Morris RI, Jackson N, Smith A, Black SA. A systematic review of the safety and efficacy of inferior vena cava stenting. Eur J Vasc Endovasc Surg. 2023;65(2):298-308.
14. Rognoni C, Furnari A, Lugli M, Maleti O, Greco A, Tarricone R. Time-driven activity-based costing for capturing the complexity of healthcare processes: the case of deep vein thrombosis and leg ulcers. Int J Environ Res Public Health. 2023;20(10):5817.