Overview on foam sclerotherapy in the treatment of varicose veins
Tomasz Urbanek,MD
Department of General Surgery,
Vascular Surgery, Angiology and
Phlebology; Medical University of
Silesia, Katowice, Poland; European
Center of Phlebology, Katowice,
Poland
ABSTRACT
Foam sclerotherapy has become an important part of the treatment for patients with chronic venous diseases (CVD), including both medical and esthetical indications. The wide implementation of foam sclerotherapy in C1 to C6 patients, together with growing clinical experience and number of performed studies, positions foam sclerotherapy as a valuable treatment method in the current CVD management guidelines as well as several consensus documents. The technological progress and improvement in good-quality foam preparation, as well as an improvement in its administration adjusted to the treated pathology, allow the achievement of satisfactory results in a variety of patients and clinical conditions. This article presents an overview of the current position of foam sclerotherapy treatment in the CVD management guidelines, together with an update on physician compounded, as well as standardized, foam preparation and administration options. The options for treatment of truncal vein incompetence followed by varicose vein/tributary and C1 foam sclerotherapy are also discussed in light of current clinical experience, technical solution availability, and study results. Foam sclerotherapy remains an important compound for phlebological treatment, as it’s often the method of choice or a complementary part of CVD patient management.
Introduction
Sclerotherapy remains a basic treatment technique in many centers treating patients with varicose veins and other chronic venous disease (CVD)-related pathologies that cause esthetic as well as often severe medical problems. Introducing foam as a method of drug administration expanded the indications and increased the efficacy of sclerotherapy in the treatment of CVD.1 In daily practice, foam is used as a treatment for a wide range of venous pathologies, from C1 to C6 class, including patients with varicose vein recurrence and patients with venous leg ulcer (VLU), and also for cosmetic indications in patients with CVD. Wide acceptance of the fact that foam formulations of the sclerosing agent is at least twice as effective as liquid, with 4 or 5 times less sclerosing agent needed, opened up new treatment possibilities.1,2 However, several limitations and precautions concerning foam administration should be considered during the planning and performance of the procedure.2
Foam sclerotherapy: foam production
and optimization of foam quality
and optimization of foam quality
In most countries, the use of foam in sclerotherapies remains based on physician-compounded foam (PCF) production. It is widely accepted and also suggested in the sclerotherapy guidelines: the use of small, good-quality bubbles in a homogeneous and viscous foam is advised.1,2
Sclerosant foam is usually generated by mixing a liquid detergent with a gas—in most cases, air. As an alternative, carbon dioxide (CO2) or a combination of CO2 and oxygen (O2) can be used.1,2 According to the Tessari method for foam creation, 2 syringes connected by a 3-way stopcock can be used with a liquid/air ratio of 1 to 4 (Figure 1A).3 Another commonly used method (the double-syringe system [DSS]) uses 2 syringes and a 2-way connector (Figure 1B).4 Irrespective of the method of foam creation, the generated foam can be classified as macrofoam (>500 µm), minifoam (250-500 µm), and the most desired, microfoam (<250 µm), depending on the bubble diameter achieved.2
Several factors have been suggested as possible influencers of foam quality, and even different foam doses prepared by the same physician can significantly differ in terms of quality.1,2,5-7 To avoid foam degradation, the time between foam creation and its clinical use should be the shortest possible, and during foam preparation, a variety of potentially related factors affecting foam stability and bubble size should be considered. This is also related to the materials used for foam production.1,2 A higher sclerosing-agent concentration allows creation of a more stable foam. Foam stability can be significantly reduced by the silicone content of the inner surface of the syringes used for foam creation.1,2 In this aspect, the syringes containing a reduced amount of silicone should be preferred. The use of alternative gases, proposed by some authors, to decrease the potential adverse events rate related to the foam can also be important.2,8,9 With respect to gas content, foam containing CO2 only is significantly less stable, leaving a shorter time for its application than standard air-based foam. One of the proposed solutions to increase CO2 foam stability is to use nitrogen-free or low-nitrogen foam based on the CO2/O2 gas mixture usage.2 As the liquid plus gas fraction remains an important factor related to foam stability, for PCF, the most commonly implemented liquid/air ratio is that of the Tessari foam (1:4), although some other combinations are also available.2 It should be emphasized that usage of foam created with macrobubbles (>500 µm) can potentially and more likely lead to cerebral artery air embolization in patients predisposed to paradoxical embolism.2
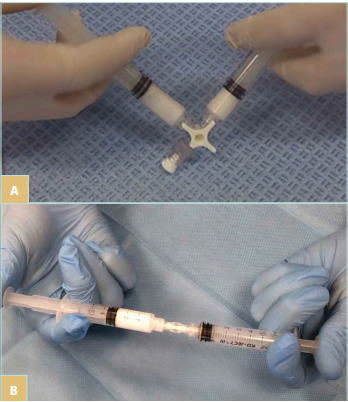
Figure 1. A) Foam preparation: 3-way stopcock and Tessari method. B) Foam preparation: double-syringe system (DSS) method.
To obtain good-quality foam via an operator-dependent technique, eg, PCF, there are a few points to consider. When using PCF, the liquid sclerosing agent is pumped back and forth between 2 connected syringes through a connector. A factor that can potentially influence foam quality and stability is the pressure within the foam creation system. To obtain good-quality foam, high pressure should be applied to both syringes compressing their content during foam creation. This can be done by appropriate finger pressure application on the syringe plungers during the pumping of the syringe content back and forth. Another modification proposed by some authors is the use of filters that can be inserted into the foam production system to increase the possibility of microbubble generation.1,2
Another factor that should not be forgotten during planning of the foam sclerotherapy procedure is the size of the needle used, as very small needles can lead to foam degradation. It is commonly accepted that for foam-based procedures, needles that are 25 G or larger (preferred) should be used in order to avoid needle-related foam destruction and degradation.1 The best-quality foam can be maintained when large needles or catheters are used.

Figure 2. Varithena (polidocanol injectable foam): 1% polidocanol and low nitrogen, O2/CO2 gas mixture. Image courtesy of F. Lurie.
As mentioned above, due to multiple factors, PCF is not only operator dependent, but also susceptible to differences in quality depending on the materials, syringes, drug concentration, or air/liquid formula used.1 Such observations open up the field to research on options for standardized foam creation that would be repeatable and operator independent.
Some solutions are already available on the market and can be mentioned here. One of these is the EasyFoam kit (Kreussler), which, however, does not exclude operator influence. The kit consists of a 10-mL, low-silicone, disposable syringe filled with the required amount of sterile air with a fixed bidirectional check valve and connector; a 5-mL low silicone disposable syringe (for injection purposes); and needles. Foam that is stable, homogeneous, viscous, and with fine bubbles can be obtained under standardized conditions; however, even if easy to handle, the system requires manual operation (foam creation) by the physician after filling the smaller syringe with the proper amount of the sclerosing agent.10 Among other products commercially available on some markets, Varithena (Boston Scientific) can be mentioned. Varithena (polidocanol injectable foam) contains 1% polidocanol and low nitrogen (<0.8%), O2/CO2 gas mixture (65:35), with a gas/liquid ratio of 7:1 (Figure 2). The Varithena system enables microfoam production with a median bubble diameter of 500 µm; the high gas/liquid ratio in the stable foam enhanced blood displacement from the treated vessels.11 The efficacy of Varithena has been confirmed in several trials.12-14 There are also a few Varithena limitations, including the fixed sclerosant concentration and sclerosant type, lack of worldwide availability, as well as a significant cost. Looking for other options, the machine supported mixing of the sclerosing agent with gas by a dedicated semiautomatic device has also been proposed. This concept aims to obtain the same number and speed of the syringe plunger movements in the DSS, which has to be introduced and fixed in the machine before gas/liquid mixing (eg, the TurboFoam device; Kreussler).15 The most recent and very promising proposal to obtain standardized and good-quality foam is the Varixio device (Automated Microfoam Preparation System, VB Devices) (Figure 3), which allows the preparation of microfoam with air or physiological gases (O2/CO2; low nitrogen 2%-10% N2) and various (also very low) concentrations of the sclerosing agent. In this standardized, automated procedure, the sclerosing agent is added to special sterile capsules containing air or gases and that are designed to produce good-quality microfoam with a bubble diameter less than 250 µm (from 84±14 to 119±6; average 100 µm), with a mean foam half-life of 5.2 minutes and gas/liquid ratio between 1:5 and 1:7. The capsules are connected to the preprogrammed magnetic stirrer machine, and foam of standard but also, if needed, with very low concentration of the sclerosing agents can be obtained (which with high quality was earlier not available for very low concentrations via the standard Tessari method protocol, eg, for 0.2% polidocanol).16 Scientific evidence with regard to this new method is currently growing.17
To summarize, the commonly seen differences in foam quality (among various physicians and centers), as well as the official sclerotherapy-drug registration issues and the fact that in some countries only some concentrations of the drugs are registered as a foam formula, encourage and stimulate further research on foam standardization. Undoubtedly, this research should be continued.
Saphenous vein foam sclerotherapy,
large vein sclerotherapy
large vein sclerotherapy
The use of sclerotherapy for treatment of saphenous vein incompetence should be based on ultrasound-guided foam sclerotherapy (UGFS) performed via direct vein puncture or catheter introduction followed by foam injection. Using direct vein puncture by needle or short catheters (always ultrasound guided), the length and size of the treated vein segment should also be taken into consideration, as mixing foam with the blood in long vein segments treated from a single vein access point can lead to procedure failure or incomplete vein closure due to sclerosing agent deactivation by blood proteins.1,2,18,19 To avoid such an issue, instead of the single vein puncture (eg, in the upper thigh in the treatment of the incompetent great saphenous vein [GSV]), multiple vein punctures with separate foam administration can be used, especially when treating longer vein segments (eg, from upper calf to the saphenofemoral junction, Figure 4). The same approach can be applied to the long incompetent small saphenous vein (SSV) segments or any other long superficial vein treated. Alternatively (especially for long incompetent GSV and SSV), catheter-directed foam sclerotherapy (CDFS) can be applied with the use of long catheters and ultrasound guidance. The option of using ultrasound-guided CDFS in saphenous vein treatment is currently also included in the recent European Society for Vascular Surgery (ESVS) guidelines (recommendation class IIB, level B).20 An analysis of evidence from 3689 patients (systematic review and meta-analysis) by Lim and coworkers suggests a higher rate of occlusion with CDFS than with UGFS in 3-year follow-up (82.4% vs 62.9%).21 According to the 2022 ESVS guidelines, in patients with GSV incompetence, first-line therapy (if anatomically feasible) remains endovenous thermal ablation in preference to surgical high ligation or UGFS (class I, level A of recommendations).20 With regard to UGFS as a method for GSV treatment, the authors of the ESVS guidelines suggest UGFS (if this method is chosen) for patients with GSV trunk diameter less than 6 mm.20 The same guidelines also prefer the use of thermal methods in treatment of SSV incompetence in preference to surgery or UGFS.20 The reason for not using foam sclerotherapy as first-line therapy for large incompetent lower-limb truncal veins is based on long-term results of GSV foam sclerotherapy. Keep in mind, however, that the lower rates of GSV occlusion in UGFS versus other (especially thermal) methods can be related to several factors, also including the way the procedure is performed (eg, one single foam injection into the thigh part of the GSV instead of repeated foam injections along the long incompetent GSV segments) and the use of foam in treatment of large and very large GSV. Rasmussen et al, in 3-year follow-up results of GSV treatment (laser ablation, radiofrequency ablation, surgery, and UGFS), documented treatment failure in 6.8% to 7% of cases after thermal ablation and 26.4% of cases after UGFS.22 After 5 years of follow-up in this study, the GSV complete occlusion rate was only 33.3%, which corresponds with previously reported 5-year results published by van der Velden et al (23% GSV occlusion rate after 5-year follow-up).23,24
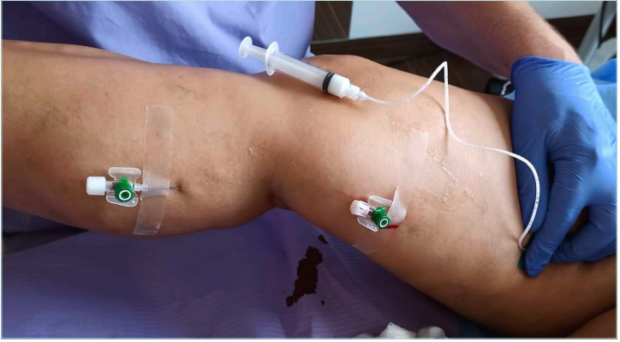
Figure 4. Great saphenous vein (GSV) sclerotherapy with multiple injections (ultrasound-guided foam sclerotherapy). GSV incompetence from the upper calf to the saphenofemoral junction.
Apart from the guidelines and the often-unsatisfactory anatomical success in long-term results, the use of UGFS in truncal vein treatment continues in many centers, at least for some indications, owing to cost-efficacy, patient satisfaction, and tolerance, as well as satisfactory postprocedure quality of life.20 Such indications include varicose vein recurrence, small vein diameter, and angulated course. The saphenous vein incompetence treatment in locations not amenable to thermal methods should also be mentioned (eg, distal calf saphenous vein segments).1,2,20 In the qualification for truncal vein UGFS, size of the treated vein should also be taken into consideration. Shadid et al, comparing results of GSV treatment in veins that were smaller or larger than 6 mm (when measured at mid-thigh), confirmed a higher 2-year reflux recurrence rate in veins over 6 mm in diameter (62.6% vs 42% for the veins less than 6 mm).25 Venermo et al, in the randomized controlled trial (RCT) comparing endovenous laser ablation (EVLA), high ligation with stripping, and UGFS in a cohort of 214 patients, documented a 1-year occlusion rate of 51% in the UGFS group (vs 97% for the thermal method and for surgery). The results of UGFS differed significantly between the subgroups of patients with veins under 6 mm in diameter (75% occlusion rate) and those with veins over 9 mm (40% occlusion rate).26
With regard to published SSV treatment results, those with UGFS remain inferior to those with thermal methods. Boersma et al, in a systematic review and meta-analysis of 49 studies including 5 RCT, documented a pooled success rate of 98.5% after EVLA (mean follow-up of 12.5 months), 94.1% for radiofrequency ablation (RFA; mean follow-up of 14.3 months), and 63.6% for UGFS (mean follow-up of 10.4 months).27 In the recent FOVELASS study (RCT Comparing EVLA Versus Polidocanol Foam in the Treatment of SSV Insufficiency), focusing on the SSV, 161 patients were randomized to EVLA or UGFS groups. According to 36-month follow-up results, the rate for lack of reflux was significantly better after EVLA (86%) than after UGFS (56%) (odds ratio [OR] 5.36; 95% CI, 2.31-12.44).28
Other possibilities for UGFS in truncal vein incompetence treatment have also been proposed. Some authors suggest that use of long catheters in UGFS for saphenous vein incompetence treatment can be supported by application of a perivenous tumescent solution.29,30 This approach allows for vein compression and successful removal of a significant amount of blood from the treated vein segment, as well as a decrease in the volume of foam used. In an RCT performed by Devereux and coworkers, comparing saphenous vein ultrasound-guided CDFS with the same treatment but supported by perivenous tumescent local anesthesia, the full 1-year occlusion rate in the tumescent anesthesia group was 73.9%, with partial occlusion in another 8.7% of the patients. However, compared with the standard treatment group, results with perivenous solution application were not better than those in the ultrasound-guided-CDFS-only approach.31 Publication of results from this study triggered some discussion, as in the Devereux study, tumescent solution that did not include adrenaline was given and a significant number of the patients were lost to follow-up (20% in the nontumescent and 8% in the tumescent group).32 In the study by Ali and coworkers, 3-year results in the group of 249 patients with GSV incompetence treated via ultrasound-guided CDFS combined with tumescent anesthesia were analyzed. Permanent obliteration of the saphenous vein after 36 months of follow-up was achieved in 81.5% GSV, and 89.6% of treated patients were free of above-knee GSV reflux.29 Similar results were presented by Cavezzi et al in a prospective observational study with 12- and 36-month GSV occlusion rates of 94.3% and 89.4% after ultrasound-guided CDFS with tumescent solution application and vein irrigation before foam administration. The median diameter of the treated GSV trunk in this study was 7.1 mm.30 Further studies are needed to confirm the benefits of tumescent solution– based vein compression on the more effective permanent saphenous vein occlusion rate when treated by UGFS. On the other hand, the significant foam volume reduction achieved with tumescent solution application can already be an interesting option for patients with large varicose veins, including varicose vein recurrence, for example, in the form of large groin neovascularization.
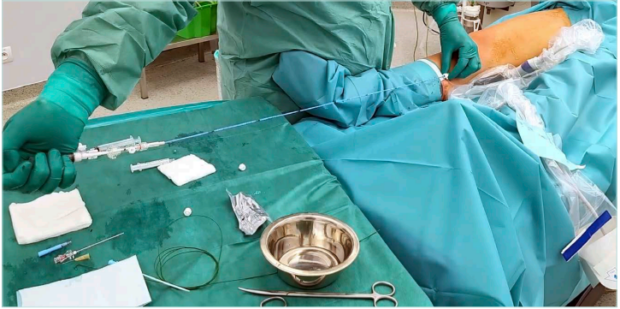
Figure 5. Foam administration by Flebogrif catheter (mechanochemical great saphenous vein ablation).
There are several observations and trials suggesting the use of UGFS together with thermal ablation. Besides the commonly used concept of truncal vein thermal ablation and saphenous vein tributary foam sclerotherapy treatment, some special treatment options have also been proposed. In the laser-assisted foam sclerotherapy (LAFOS) technique, proposed by Frullini and Fortuna, the specially designed laser Ho:YAG (Holmium:Yttrium-Aluminium-Garnet) 2100-nm ablation is used to shrink the vein immediately before foam administration, which allows the use of a smaller amount of foam and no tumescent anesthesia.33 Sclerofoam-assisted laser therapy (SFALT) is another technique proposed by Italian authors.34 For this technique, a 1470-nm laser with radial fiber is used. Initially, a short occlusion of the saphenous vein 1 cm below the superficial epigastric vein is created by laser ablation. After creation of this shrunk plug in the proximal saphenous vein segment, 1% polidocanol or 1% sodium tetradecyl sulfate (STS) foam is administered, causing the vein to shrink, which is followed by laser ablation with a significant reduction in the usual energy fluence. The authors of this method did not use tumescent anesthesia (except its use in the proximal 1-cm saphenous vein laser ablation); however, mild intravenous sedation was used in the treated patient cohort.34 Among the other proposed foam-based treatment options, use of long sheaths for endovenous laser fiber introduction as well as for local foam application (eg, into groin neovascularization) followed by standard truncal laser ablation in the GSV segment below can be mentioned. Some technical solutions with special designed laser fibers and an additional injection canal (designed originally by the fiber inventors for vein saline flushing) are now also available.35
Foam application can also be part of the mechanochemical ablation. This concept is used for the Flebogrif catheter (Balton) designed for truncal vein mechanochemical ablation (Figure 5). The specially designed tip of the catheter, with hooks irritating the vein wall (and cutting the internal layer of the vein wall) after catheter-tip opening, provokes vein spasm, which is followed by direct foam application during catheter pullback in the treated veins. In a study based on 200 treated patients with GSV incompetence, its Polish authors documented a 92% 24-month follow-up success rate.36 The efficacy of this device is currently being tested in new clinical trials—further studies, including long-term follow-up studies need to be performed to define the group of the patients with GSV incompetence that would benefit most from this procedure. In another mechanochemical ablation system available on the market (Clarivein, Merit Medical), a liquid sclerosing-agent solution is administered with another rotational mechanism, leading to vein-wall spasm. Another commercially available concept focusing on the potential increase in UGFS efficacy is the aspiration infusion kit (Sclerosafe, VVT Medical Ltd) dedicated to foam application with simultaneous blood aspiration from the vein lumen via a specially designed catheter and double syringe kit. Despite the interesting concept, until now, only evidence from a small patient series with limited follow-up has been available.37
Tributaries, varicose veins, and small-vein foam
sclerotherapy
sclerotherapy
Foam sclerotherapy is an interesting and efficient alternative to surgical phlebectomy/miniphlebectomy procedures in the incompetent tributaries, as well as to varicose vein treatment, including both saphenous- and nonsaphenous-related ones. The choice of the sclerosing-agent concentration used for tributaries/varicose vein sclerotherapy depends on the size of the treated veins (Table I).
Visible varicose veins and visible tributaries can be treated with vein access under visual control (with blood aspiration to confirm needle presence in vein lumen). In some of these cases, especially for large veins, many reflux sources and complex pathology, or for veins that are not well visible, UGFS can be a valuable option. To facilitate mid- and small-size vein punctures, other vein visualization technologies can be used, including transillumination or near infrared imaging (NIR) (Figures 6 and 7). Recent advancement in ultrasound technology (especially high-frequency ultrasound) also allows the diagnosis and treatment of very small reticular or feeding veins under ultrasound guidance (Figure 8).
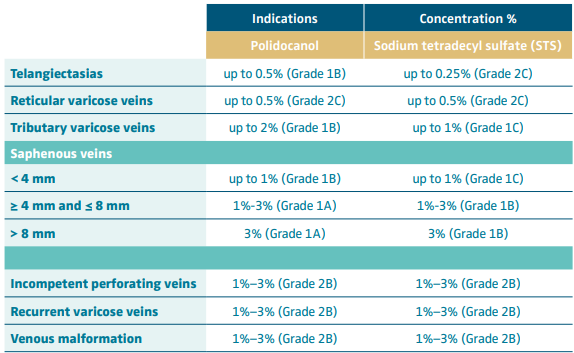
Table I. Suggested polidocanol and sodium tetradecyl sulfate concentrations in foam sclerotherapy according to the European Guidelines for Sclerotherapy with grade of recommendations.
Based on reference 1: Rabe et al. Phlebology. 2014;29:338-354.
To achieve the best possible results, proper preoperative reflux mapping and elimination of the reflux sources in primary as well as recurrent varicose veins should always be incorporated.1,2,20 In patients with tributary incompetence related to saphenous trunk incompetence, the tributary treatment (by sclerotherapy or miniphlebectomy) can be performed as part of the concomitant or staged treatment.20 In the latter approach, tributary foam sclerotherapy is performed during a separate procedure after a previous saphenous vein ablation or surgery. Following general rules concerning sclerotherapy of the tributary, varicose veins, or small veins, treatment from proximal to distal leakage points and from larger to smaller varicose veins is suggested.1
For performance of the procedure, smooth-moving syringes with slow intravenous foam injection, as well as multiple injections, are proposed.1,2 In many cases including patients with more extensive pathology, repeated sessions may be necessary. According to current ESVS guidelines, “For patients with CVD requiring treatment of varicose tributaries, ambulatory phlebectomy, UGFS or a combination of both are recommended.”20 Choosing the proper techniques depends largely on a physician’s experience and preference in terms of a patient’s expectations and should be individually discussed. In patients looking for a good cosmetic outcome, with large and very superficially located tributaries, as well as in patients with unaccepted hyperpigmentation after previous sclerotherapy, ambulatory phlebectomy can be chosen as a treatment option and should be individually discussed with the patient.1,2,20 In patients with advanced trophic skin changes, performance of phlebectomies may be affected by the number of local complications, which makes UGFS a valid alternative option.20 According to the EVRA study (A Randomized Trial of Early Endovenous Ablation in Venous Ulceration)38 and daily practice, successful reflux ablation increases the rate of healed venous leg ulcers (VLU). One of the most important parts of successful venous hypertension elimination is not only the truncal or tributary as well as varicose vein treatment but also elimination of the reflux in the subulcer VLU plexus, which is identified in many VLU patients.1,39
The discussion concerning the amount of foam that can be used during one sclerotherapy session is still open. According to the European guidelines for sclerotherapy, experts suggest that during routine procedures the amount of foam injected should not exceed 10 mL, and in cases where a larger amount is considered, an individual risk-benefit evaluation should always be undertaken.1 Among the factors that need to be evaluated, vein location and its direct connection to the deep vein system should be mentioned (eg, proximal part of the truncal vein). The Australasian College of Phlebology Standards guidelines suggest use of 10 mL of foam for truncal vein incompetence and (in a separate session) up to 15-20 mL for tributaries (provided foam is not noted on ultrasound to extend into the deep system).40 According to the recent ESVS guidelines, a foam volume limit of up to 16 mL is suggested, which (with low level of evidence) complies with European regulations.41 Further studies are needed to establish the maximum and safe amount of foam for various kinds of sclerotherapy.
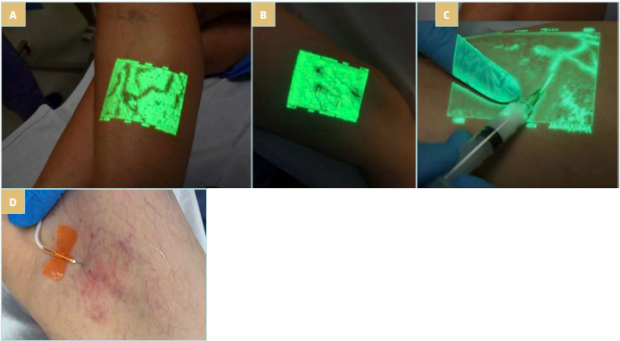
Figure 7. A,B) Near infrared (NIR) imaging in vessel identification during sclerotherapy procedure: foam sclerotherapy under NIR guidance. C) NIR imaging visualization–based procedure with C1 feeding vein identification and low-concentration foam sclerotherapy. D) Foam sclerotherapy of feeding veins in C1 patient.
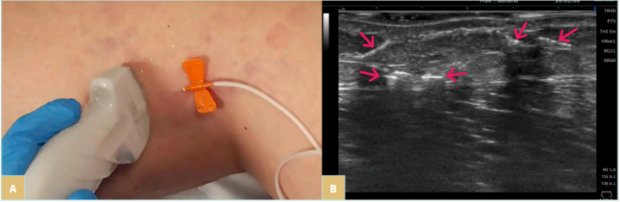
Figure 8. A,B) High-frequency ultrasound-guided C1 foam sclerotherapy (foam visible in the feeding vessels).
The beneficial effects of sclerotherapy in treating tributaries and nonsaphenous varicose veins have been widely described in the literature.42-45 Currently, foam is also used in small vein (C1) treatment; however, the formula used for proper application as well as drug concentration remain subjects of continuous discussion. According to the European Guidelines for sclerotherapy in CVD, in C1 pathology, both liquid and foam treatment can be applied.1 Like in other CVD patients, also in this case, potential local complications related to administration of the sclerosing agent, including hyperpigmentation and matting, need to be taken into consideration. Apart from patient-related factors (eg, previous hyperpigmentation or matting, skin type, estrogen, or other medical therapy exposure, as well as concomitant condition presence), administration of an agent that is too strong, high pressure during injection, as well as treatment of large areas with a single injection may have a potential role in the occurrence of this complication. As previously suggested, foam is usually much more potent than liquid.20 This leads to the suggestion that in C1 pathology treatment, a very low foam concentration for sclerotherapy should be used (according to the European Guidelines for sclerotherapy, up to 0.5% polidocanol and up to 0.25% STS in telangiectasia treatment, and up to 0.5% polidocanol and STS for reticular veins was proposed).1 Besides occurrence of the various possible local pathologies in C1 patients (from simple telangiectasia to complex reticular veins or difficult-to-identify feeding veins), the issues related to low-concentration, good-quality foam creation should also be mentioned. Using standard PCF with a very low sclerosing agent concentration (eg, 0.25% polidocanol, 0.2% or lower STS), low-quality foam can usually be obtained, which is also potentially degraded by the use of very small needles. Another factor to potentially take into consideration is that in some countries, the lowest sclerosing-agent concentration is not registered as suitable for foam applications, which makes foam sclerotherapy in C1 patients an off-label approach. Despite these facts, the use of a low-concentration foam remains an interesting alternative for liquid sclerotherapy in C1 pathology, which is especially effective in complex reticular vein treatment. The problem of low-concentration foam stability can potentially be overcome with the new automated foam creation modalities.46 In the discussion of a potential skin hyperpigmentation risk in patients undergoing C1 foam sclerotherapy, the systematic review performed by Bossart and coworkers should be mentioned.47 According to this systematic review, there is a comparable incidence of hyperpigmentation for 0.25% polidocanol in liquid and foam. Two available direct comparison studies show no differences48 or liquid superiority49; however, the second of these studies was based on a very limited number of patients (20 cases).48,49 The authors of the systematic review emphasized that the rate of hyperpigmentation grows in accordance to the concentration of polidocanol in C1 pathology treatment for both liquid and foam (from 2%-25% for 0.25% polidocanol to 13%-73% for 1% polidocanol—liquid and foam).47
Conclusions
The use of foam has become standard of care in many CVD related pathologies. Knowledge of treatment limitations, potential contraindications, as well as complications of foam sclerotherapy treatment should be an integral part of phlebological education as well as sclerotherapy planning.1,2 The absolute contraindications to foam sclerotherapy (hypersensitivity to sclerosing agent, acute venous thromboembolism, severe neurological or cardiac adverse events including known symptomatic patent foramen ovale [PFO], acute systemic illness, infection or uncontrolled chronic disease, or severe peripheral arterial disease) should be accepted, but knowledge about several relative contraindications is also mandatory.1,2 Length restrictions for the current article do not allow for discussion of all possible foam sclerotherapy complications, but knowledge of possible sclerotherapy complications, also concerning complications that are more commonly seen in patients treated with foam versus liquid (eg, visual disturbances, headache, and migraine), is required and should be continuously updated on the basis of previously published documents and new publications (see References 1 and 2).

CORRESPONDING AUTHOR
Prof Tomasz Urbanek, MD
Department of General Surgery, Vascular
Surgery, Angiology and Phlebology,
Medical University of Silesia, Katowice,
Poland
European Centre of Phlebology,
Katowice, Poland
EMAIL: sandeeprajpandey@gmail.com
References
1. Rabe E, Breu FX, Cavezzi A, et al. European guidelines for sclerotherapy in chronic venous disorders. Phlebology. 2014;29:338-354.
2. Wong M, Parsi K, Myers K, et al. Sclerotherapy of lower limb veins: indications, contraindications and treatment strategies to prevent complications – a consensus document of the International Union of Phlebology-2023. Phlebology. 2023;38:205-258.
3. Tessari L, Cavezzi A, Frullini A. Preliminary experience with a new sclerosing foam in the treatment of varicose veins. Dermatol Surg. 2001;27:58-60.
4. Rao J, Goldman MP. Stability of foam in sclerotherapy: differences between sodium tetradecyl sulfate and polidocanol and the type of connector used in the double syringe system technique. Dermatol Surg. 2005;31:19-22.
5. Meghdadi A, Jones SA, Patel VA, Lewis AL, Millar TM, Carugo D. Foam-in-vein: a review of rheological properties and characterization methods for optimization of sclerosing foams. J Biomed Mater Res B Appl Biomater. 2021;109:69-91.
6. Roberts TG, Cox SJ, Lewis AL, Jones SA. Characterisation and optimisation of foams for varicose vein sclerotherapy. Biorheology. 2020;57:77-85.
7. Cavezzi A, Tessari L. Foam sclerotherapy techniques: different gases and methods of preparation, catheter versus direct injection. Phlebology. 2009;24:247-251.
8. Morrison N, Neuhardt DL, Rogers CR, et al. Comparisons of side effects using air and carbon dioxide foam for endovenous chemical ablation. J Vasc Surg. 2008; 47:830-836.
9. Morrison N, Neuhardt DL, Rogers C, et al. Incidence of side effects using carbon dioxide-oxygen foam for chemical ablation of superficial veins of the lower extremity. Eur J Vasc Endovasc Surg. 2010;40:407- 413.
10. Rabe E, Otto J, Schliephake D, Pannier F. Efficacy and safety of great saphenous vein sclerotherapy using standardised polidocanol foam (ESAF): a randomised controlled multicentre clinical trial. Eur J Vasc Endovasc Surg. 2008;35:238-245.
11. Varithena website. Home page. https:// www.varithena.com/en-us/home.html
12. Gibson K, Kabnick L; Varithena® 013 Investigator Group. A multicenter, randomized, placebo-controlled study to evaluate the efficacy and safety of Varithena® (polidocanol endovenous microfoam 1%) for symptomatic, visible varicose veins with saphenofemoral junction incompetence. Phlebology. 2017;32:185-193.
13. Todd KL 3rd, Wright DI; VANISH-2 Investigator Group. Durability of treatment effect with polidocanol endovenous microfoam on varicose vein symptoms and appearance (VANISH-2). J Vasc Surg Venous Lymphat Disord. 2015;3:258-264.
14. Star P, Connor DE, Parsi K. Novel developments in foam sclerotherapy: focus on Varithena (polidocanol endovenous microfoam) in the management of varicose veins. Phlebology. 2018;33:150-162.
15. Blaise S, Bosson JL, Diamand JM. Ultrasound-guided sclerotherapy of the great saphenous vein with 1% vs. 3% polidocanol foam: a multicentre double blind randomised trial with 3-year follow up. Eur J Vasc Endovasc Surg. 2010;39:779- 786.
16. Varixio website. Home page. www.varixio. com/professionals
17. Alongi G, Bissacco D, Cervi E. Three-year follow-up analysis of automated microfoam preparation system for great saphenous vein incompetence and varicose veins sclerotherapy treatment. Phlebology. 2024;39(7):471-476.
18. Watkins MR. Deactivatio of sodium tetradecyl sulphate injection by blood proteins. Eur J Vasc Endovasc Surg. 2011;41:521-525.
19. Bottaro E, Paterson JAJ, Quercia L, et al. In vitro and ex vivo evaluation of the biological performance of sclerosing foams. Sci Rep. 2019;9:9880.
20. De Maeseneer MG, Kakkos SK, Aherne T, et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2022 Clinical practice guidelines on the management of chronic venous disease of the lower limbs. Eur J Vasc Endovasc Surg. 2022;63:184- 267.
21. Lim SY, Tan JXD, D’Cruz RT, Syn N, Chong TT, Tang TY. Catheter-directed foam sclerotherapy, an alternative to ultrasound guided foam sclerotherapy for varicose vein treatment: a systematic review and meta analysis. Phlebology. 2020;35:369-383.
22. Rasmussen L, Lawaetz M, Serup J, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy, and surgical stripping for great saphenous varicose veins with 3-year follow-up. J Vasc Surg Venous Lymphat Disord. 2013;1:349-356.
23. Brittenden J, Cooper D, Dimitrova M, et al. Five-year outcomes of a randomized trial of treatments for varicose veins. N Engl J Med. 2019;381:912-922.
24. van der Velden SK, Biemans AA, De Maeseneer MG, et al. Five-year results of a randomized clinical trial of conventional surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy in patients with great saphenous varicose veins. Br J Surg. 2015;102:1184-1194.
25. Shadid N, Nelemans P, Lawson J, Sommer A. Predictors of recurrence of great saphenous vein reflux following treatment with ultrasound-guided foam sclerotherapy. Phlebology. 2015;30:194-199.
26. Venermo M, Saarinen J, Eskelinen E, et al. Randomized clinical trial comparing surgery, endovenous laser ablation and ultrasound-guided foam sclerotherapy for the treatment of great saphenous varicose veins. Br J Surg. 2016;103:1438-1444.
27. Boersma D, Kornmann V, van Eekeren R, et al. Treatment modalities for small saphenous vein insufficiency: systematic review and meta-analysis. J Endovasc Ther. 2016;23:199-211.
28. Hamel-Desnos C, Nyamekye I, Chauzat B, Gracia S, Josnin M, Abbadie F. FOVELASS: a randomised trial of endovenous laser ablation versus polidocanol foam for small saphenous vein incompetence. Eur J Vasc Endovasc Surg. 2023;65:415-423.
29. Ali H, Elbadawy A, Saleh M, Mahmoud O. Mid-term results of catheter directed foam sclerotherapy combined with tumescent local anaesthesia for treatment of great saphenous vein incompetence. Eur J Vasc Endovasc Surg. 2017;54:363-368.
30. Cavezzi A, Mosti G, Campana F, Tessari L, Bastiani L, Urso SU. Catheter foam sclerotherapy of the great saphenous vein, with perisaphenous tumescence infiltration and saphenous irrigation. Eur J Vasc Endovasc Surg. 2017;54:629-635.
31. Devereux N, Recke AL, Westermann L, Recke A, Kahle B. Catheter-directed foam sclerotherapy of great saphenous veins in combination with pre-treatment reduction of the diameter employing the principals of perivenous tumescent local anesthesia. Eur J Vasc Endovasc Surg. 2014;47:187-195.
32. Cavezzi A, Mosti G, Di Paolo S, Tessari L, Campana F, Urso SU. Re: ‘Catheter-directed foam sclerotherapy of great saphenous veins in combination with pre-treatment reduction of the diameter employing the principals of perivenous tumescent local anesthesia.’ Eur J Vasc Endovasc Surg. 2014;48:597.
33. Frullini A, Fortuna B. Laser assisted foam sclerotherapy (LAFOS): a new approach to the treatment of incompetent saphenous veins. Phlebologie. 2013;66(1):51-54.
34. Zini F, Tessari L, Torre R. Sclerofoam assisted laser therapy for saphenous refluxes: an innovative tumescence-free technique. Veins Lymphatics. 2015;4:5141. https:// doi.org/10.4081/vl.2015.5141
35. vascularnews. Biolitec reveals new ELVeS Radial 2ring Pro laser fibre for severely tortuous veins. Published February 3, 2020. www.vascularnews.com/biolitec-reveals new-elves-radial-2ring-pro-laser-fibre-for severely-tortuous-veins/
36. Iłżecki M, Terlecki P, Przywara S, Iłżecka J, Dave S, Zubilewicz T. The novel minimally invasive mechano-chemical technique of the saphenous vein ablation. Our center experience: results of 24 months follow-up. Acta Angiologica. 2019;25:127-132.
37. Kolvenbach R, Olami H, Brandeis Z. Retrospective evaluation of safety and efficacy for sclerosafe device – case report summary. www.i-t-d.de/download/Case_ Report_Summary_for_ScleroSafe.pdf
38. Gohel MS, Heatley F, Liu X, et al; EVRA Trial Investigators. A randomized trial of early endovenous ablation in venous ulceration. N Engl J Med. 2018;378:2105-2114.
39. Bush R. New technique to heal venous ulcers: terminal interruption of the reflux source (TIRS). Perspect Vasc Surg Endovasc Ther. 2010;22:194-199.
40. Australasian College of Phlebology. Ultrasound guided sclerotherapy: diagnose venous disease and treat superficial venous incompetence with injected sclerosants under ultrasound guidance. www.phlebology.com.au/newsletter_docs/ ACPUGSStandard2016.pdf
41. European Medicine Compendium. Summary of Product Characteristics for Fibrovein 3% solution for injection. https://www. medicines.org.uk/emc/product/1199/ smpc#gref
42. Zhang J, Jing Z, Schliephake DE, Otto J, Malouf GM, Gu YQ. Efficacy and safety of Aethoxysklerol® (polidocanol) 0.5%, 1% and 3% in comparison with placebo solution for the treatment of varicose veins of the lower extremities in Chinese patients (ESA-China Study). Phlebology. 2012;27:184-190.
43. Michaels JA, Campbell WB, Brazier JE, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).Health Technol Assess. 2006;10:196.
44. de Roos KP, Nieman FH, Neumann HA. Ambulatory phlebectomy versus compression sclerotherapy: results of a randomized controlled trial. Dermatol Surg. 2003;29:221-226.
45. Vasquez M, Gasparis AP. A multicenter, randomized, placebo controlled trial of endovenous thermal ablation with or without polidocanol endovenous microfoam treatment in patients with great saphenous vein incompetence and visible varicosities. Phlebology. 2017;32:272-281.
46. Roche E, Pons R, Roche O, Puig A. A new automated system for the preparation of sclerosant foam: a study of the physical characteristics produced and the device settings required. Phlebology. 2020;35(9):724-733.
47. Bossart S, Daneluzzi C, Cazzaniga S, et al. Skin hyperpigmentations after sclerotherapy with polidocanol: a systematic review. J Eur Acad Dermatol Venerol. 2023;37(2):274-283.
48. Kern P, Ramelet AA, Wutschert R, Bounameaux H, Hayoz D. Single-blind, randomized study comparing chromated glycerin, polidocanol solution, and polidocanol foam for treatment of telangiectatic leg veins. Dermatol Surg. 2004;30:367-372.
49. Benigni JP, Sadoun S, Thirion V, Sica M, Demagny A, Chahim M. Télangiectasies et varices réticulaires. Traitement par la mousse d’Aetoxysclérol à. 0,25%. Présentation d’une étude pilote. Phlébologie. 1999;52:283-290.