Microcirculatory alterations in chronic venous disease: observation from C0s to C5 patients (CEAP classification)
Carlos Eduardo Virgini-Magalhães MD, PhD
Vascular and Endovascular
Department, State University of
Rio de Janeiro, Brazil
Eliete Bouskela, MD, PhD
Laboratory for Clinical and
Experimental Research on Vascular
Biology – BioVasc, State University
of Rio de Janeiro, Brazil
ABSTRACT
Over the last few decades, knowledge of microcirculation has expanded in its pathophysiological and molecular bases and its correlations with clinical practice. By observing images of the cutaneous circulation, it is possible to identify the degree of microangiopathy and associate its findings with the evolution of chronic venous disease (CVD). With the progression of venous disease, the cutaneous capillaries lose their typical hairpin shape of healthy individuals and early stages of the disease, become progressively enlarged and tortuous, and form masses or tangles described as glomerulus like capillaries. In more advanced stages of CVD, there is a reduction in functional capillary density. This reduction in the number of capillaries begins to be observed in C3 and is more important in classes C4, C5, and C6. The result is the replacement of healthy capillaries by large coiled vascular masses, reduced skin perfusion, and the replacement of healthy connective tissue by a chronic fibrosing inflammatory process characteristic of lipodermatosclerosis found in class C4, which eventually leads to skin ulceration. There is currently a consensus that these evolutionary changes in microcirculation are caused by the association of different hemodynamic forces and an intricate inflammatory cascade that results in a vicious cycle of proteolytic remodeling of the venous wall and further inflammation, as well as degradation of the protective endothelial glycocalyx, producing a broad spectrum of clinical symptoms ranging from varicose veins to venous ulcers.
Introduction
Since the work of Fagrell,1 in the late 1980s, the role of microcirculation in chronic venous disease (CVD) has been gradually revealed. Over the last few decades, the knowledge of microcirculation has expanded in its pathophysiological and molecular bases and, above all, in its correlations with clinical practice.
By observing images of the cutaneous circulation, it is possible to identify the degree of microangiopathy and associate its findings with the evolution of CVD. The aim of this work is to describe the interfaces between the microcirculatory changes observed in CVD and their correlation with clinical practice.
CEAP classification
The clinical-etiological-anatomical-pathophysiological (CEAP) classification for CVD, created 30 years ago, has become a universally accepted tool for describing patients in clinical practice and ensuring the standardization of scientific literature. Throughout this period, it was reviewed and updated at different times.2,3 The assessment of the 4 dimensions of CEAP is fundamentally based on information from history and accurate clinical examination, and ultrasound findings. CEAP is a descriptive tool. Although established, CEAP is also the target of criticism that includes the lack of precise definitions and reproducibility.2
The C (clinical) domain of CEAP is by far the most used in clinical practice and research. Each category describes a specific moment of CVD, it is not associated with the progression of the disease; although tempting, CEAP should not be used to assess the evolution of CVD in an individual. Therefore, it is not possible to guarantee that in the natural history of an individual’s disease, class progression will occur over time. For example, there are patients considered C1 who will never become C2 over the years. Therefore, when we describe the different clinical classes of CEAP, we limit ourselves to discussing that specific clinical picture. Several instruments are more effective for objectively measuring the evolution of the disease, as well as the results of different therapeutic approaches.
Microcirculation, a dimension completely unrelated to the aspects addressed by CEAP, can be assessed using different instruments generally used in clinical research. Despite the knowledge about the existence of microcirculatory changes in CVD, their importance in diagnosing the severity and progression of the disease is not yet considered in clinical practice. Although pathophysiology has been included in CEAP with its constant updates, we consider that detailed microcirculatory analysis should, at some point, be part of the classification owing to its importance in understanding CVD.
Microcirculatory evolution in CVD
Microcirculation is the term used to describe vessels with an internal diameter of less than 100 µm. Several methods have been used to study the microcirculation directly or indirectly, such as laser Doppler flowmetry, plethysmography, fluorescence videomicroscopy, orthogonal polarization spectral (OPS) imaging technique, and more recently the superb microvascular imaging (SMI) technique.
Microcirculatory changes in CVD have been known since the end of the last century. By observing images of the cutaneous circulation, it is possible to identify the degree of microangiopathy and associate the findings with the severity of CVD.
Cutaneous capillaries are extensions of a network composed of 2 to 4 parallel layers of arterioles and venules organized beneath the skin. They are shaped like a hairpin and are positioned perpendicular to the surface of the skin within the dermal papillae.4 The imaging tests used, such as videomicroscopy or CytocamTM, usually obtain images only of the most superficial segment of the capillary, the capillary loop. As venous disease progresses, cutaneous capillaries lose their normal hairpin-like appearance, become progressively enlarged and tortuous, and form masses or kinks described in the literature as glomerulus-like capillaries.1 In addition to capillary remodeling, there is also hypertrophy of the dermal papillae.5 These vessels of aberrant morphology with dilated interendothelial pores and irregularity of the vascular wall present increased permeability to large molecules and blood cells such as fibrinogen, macrophages, red blood cells, and plasma. It has already been suggested that the dilatation of the dermal papillae in CVD may be translated into the beginning of interstitial fluid extravasation, corresponding to a preclinical phase of limb edema found from C3.1,6
In more advanced stages of CVD, there is a reduction in functional capillary density, ie, a reduction in the number of capillaries that nourish the skin. The result is the replacement of healthy capillaries by large coiled vascular masses (Figure 1), reduced skin perfusion, and replacement of healthy connective tissue by a chronic fibrosing inflammatory process that affects the subcutaneous cellular tissue, eventually leading to skin necrosis.7
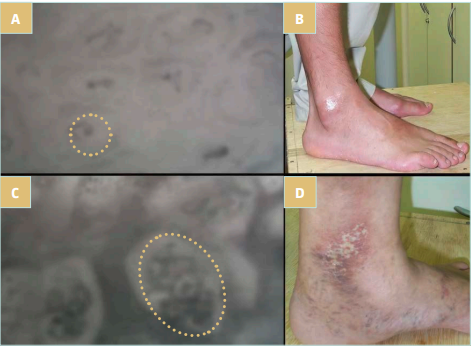
Figure 1. These images show the appearance of microcirculation in individuals with CEAP classification C0 (A and B) and C5 (C and D). The dashed circles (A and C) represent the dermal papilla, the functional cutaneous unit. Observe the difference in size of the dermal papilla and the capillary inside it in the 2 individuals. The normal capillary (A) transforms into a large vascular coil (C). Images courtesy of Virgini-Magalhães CE.
It has been suggested that microangiopathy precedes trophic skin changes and even in areas of normal skin, it is possible to observe morphological changes in capillaries with experimental methods at different stages of CEAP. No microcirculatory parameter alone can discriminate between different CEAP classes. There is an overlap of findings between classes and low interobserver reproducibility, depending on the imaging method used. In any case, microcirculation is likely to represent the bridge between macroscopic and clinical events and molecular biology.8
A study using electron microscopy analyzed the ultrastructure of the wall and venous valves of the great saphenous vein (GSV) in different CEAP classes. The authors observed that with the progression of the disease, the elastin content appears to increase, and it is possible to observe progressive damage to the endothelium of the wall and the venous valve in a similar way, with areas of absent endothelial cells and denuded basement membrane.9 There is also vascular wall heterogeneity with alternation of atrophic and hyperplastic areas, disorganized patterns of elastin layers, and micro herniations of smooth muscle cells.4
In another line of research, the group led by Professor Van Rij has been studying the role of the network of small cutaneous veins less than 2 mm in diameter in the clinical expression of CVD. Initially, a study using resin casts of amputated limbs revealed that valvular incompetence may exist in valves of small GSV tributary veins in stages C0 and C1, even though no truncal reflux can be observed on Doppler.10 More recently, a new technology known as SMI has obtained better images than color Doppler to evaluate reflux of these microvalves located in the most superficial layer of the skin surface.10,11
Previously, it was believed that these small veins did not have valves. However, the studies by Van Rij and coworkers point to some interesting aspects that could change our view about the pathophysiology of CVD. According to these authors, valve incompetence may exist even in small veins of the skin regardless of valve competence in GSV and its tributaries. In practice, many veins of the subcutaneous venous system present degenerative changes, in the absence of truncal reflux detectable on duplex scan or in the presence of clinical varicose veins. On the other hand, when there is GSV reflux, these small microvalves would be able to prevent reflux into the skin, playing a critical role in the progression of cutaneous changes in venous insufficiency.11 Such findings suggest an important role for this system of small subcutaneous veins in the CVD pathophysiology.
Remodeling of microcirculation and macrocirculation
Microcirculation is the term used to describe vessels with an internal diameter of less than 100 µm. In the skin, capillaries are mainly located in the superficial papillary layer of the dermis where venules and arterioles are organized in a composite network formed by 2 to 4 parallel and superimposed layers with the purpose of guaranteeing thermoregulation.4
There is currently a consensus that the pathophysiology of CVD has an important inflammatory component, at its origin or in the progression of the disease. The purely mechanical view that valve insufficiency is the sole cause of stasis and venous hypertension and the origin of CVD may not be sufficient to explain all aspects of its pathophysiology.10,11
The remodeling of macrocirculation and microcirculation depends in part on the strength of the hemodynamic insult and its effects on the genetic resilience of each individual.12 However, the common factor in all theories is the perpetuation of a chronic inflammatory process, which insidiously causes venous macrocirculation and microcirculation remodeling and extends to the skin and subcutaneous cellular tissue in more advanced stages of the disease.
This process involves an intricate inflammatory cascade with the participation of proinflammatory factors including reactive oxygen species (ROS), metalloproteinases (MMPs), and also adhesion molecules such as E-selectin, P-selectin, and von Willebrand factor, released by the endothelium, which in turn attract and stimulate neutrophils and platelets, and progressively cause damage to the vascular wall and extracellular matrix.4,13 Macrophages/monocytes and mast cells play an important role in this inflammatory cascade. These cells release proteolytic enzymes and ROS, participating in damage to the vascular wall.14 On the other hand, growth factors cause migration, proliferation, and dedifferentiation of smooth muscle cells that stimulate the formation of neointima and vascular remodeling.15 The result is a vicious cycle of proteolytic remodeling of the venous wall and further inflammation, as well as degradation of the protective endothelial glycocalyx, resulting in a wide spectrum of clinical symptoms ranging from varicose veins to venous ulcers.
Using the OPS technique, it is possible to obtain noninvasive images of the cutaneous microcirculation in patients with CVD and compare its findings between different CEAP classes. The more advanced the clinical class, the more changes are observed in the cutaneous capillary, which grows and takes on an atypical shape. These observations had already been published by Howlader and coworkers using videomicroscopy in different CEAP classes.16 As CVD progresses, the cutaneous capillaries with their usual hairpin-shaped appearance are being replaced by increasingly larger, glomerulus-like, coiled vascular masses. This altered morphology is found in 20% of capillaries in C1 patients and becomes increasingly common as the CEAP class advances, reaching 75% of capillaries in C5 patients (Table I). The remodeling involves the dermal papilla, a functional cutaneous unit that doubles in size when comparing patients C1 and C5.
Besides normal capillaries being replaced by large vascular coils, a gradual reduction in functional capillary density is observed, ie, the number of active capillaries decreases on the skin surface. C1 and C2 individuals have approximately the same number of functioning capillaries when compared with healthy individuals, around 20 capillaries/mm2. As the CEAP class advances, a gradual reduction is observed, where only 12 capillaries/mm2 are counted in C5.5 In more advanced stages of the disease, therefore, we find a scarce cutaneous capillary network with vessels of completely anomalous morphology.
In more advanced stages of CVD, the chronic inflammatory process extends to the surrounding tissues, subcutaneous layers, and skin. Elevated levels of MMPs in these chronically inflamed tissues contribute to excessive degradation of the extracellular matrix and collagen, which can lead to impaired healing and skin ulceration.17 The development of subcutaneous fibrosis can occur due to high levels of growth factors produced by activated leukocytes, which stimulate the excess production of fibrinogen and collagen, causing scarring fibrosis. Finally, the degradation of extravasated red blood cells and the subsequent release of hemoglobin and iron into the surrounding structures increases the oxidative state of the tissue, increasing the activity of MMPs, exacerbating tissue damage and further impairing wound healing.18
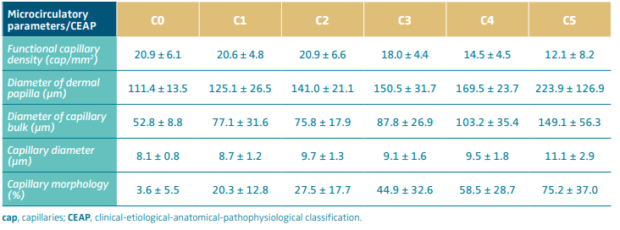
Table I. Observe changes in the measurements of microcirculatory parameters as the CEAP classification progresses. Although there are overlapping values, it is possible to observe that as the severity of the disease increases, the functional capillary density decreases at the same time as the dermal papilla and capillary vessel grow, transforming into a large, coiled vascular mass.
Based on reference 5: Virgini-Magalhães et al. J Vasc Surg. 2006;43:1037-1044.
C0s and C1 patients
The CEAP C0 class included individuals who did not present visible signs or symptoms of CVD in the lower limbs, that is, individuals with no venous disease. Even so, a significant proportion of these individuals chronically experience complaints typical of venous hypertension. CEAP incorporated the C0s nomenclature, clearly recognizing that some individuals present characteristic symptoms of CVD, even if they do not present objective visible signs of venous disease. In recent years, this clinical picture has been defined under different names: hypotonic phlebopathy, phlebopathic diathesis, prevaricose syndrome, functional phlebopathy, functional chronic venous insufficiency, or functional venous disease.
In epidemiological studies, the prevalence of C0s patients ranges from 13.9% to 19.7% of the general population.19 Complaints of numbness, burning sensation or leg heaviness, edema, muscle cramps, or restless legs may suggest the condition, especially when associated with heat, long periods of standing, specific situations such as pregnancy, type of occupation, and some seasons of the year such as spring or summer. On the other hand, resting and limb elevation tend to improve symptoms. The difficulty in diagnosis lies in the low specificity of the symptoms and the absence of clinical or imaging signs. Although some patients may or may not present permanent changes on echo-Doppler, as already described in the literature, most individuals do not present objective data to corroborate the diagnosis.3,20
The absence of specific diagnostic instruments and the lack of longitudinal studies evaluating individuals diagnosed as C0s bring uncertainty in the diagnosis and early therapeutic management of these patients, even though in the literature the use of venoactive drugs is recognized as an effective approach in controlling the symptoms in this condition.20 Considering the anti-inflammatory and antioxidant properties, platelet inhibition, improvement of endothelial homeostasis, leukocyte-endothelium interaction, among other actions, flavonoids, saponins, and some plant extracts target exactly the steps involved in the pathophysiology of symptoms and evolution of CVD even in early stages, such as in C0s patients.15
At the microcirculatory level, C0 individuals have capillaries with normal luminal diameters and dermal papillae of usual sizes. Capillary morphology is the typical hairpin shape, although a small percentage of capillaries present different degrees of coiling, characteristic of more advanced stages of CVD.5 These findings are not sufficient to ensure an adequate distinction between healthy individuals and patients in initial stages of CVD. Therefore, microcirculatory imaging parameters in C0s patients are not very different from healthy individuals when evaluated by currently available imaging research instruments.5,21
A study in the early 2000s attempted to demonstrate functional changes in the microcirculation even before the onset of capillary remodeling in CVD. Using photoplethysmography and strain-gauge plethysmography, the authors suggest hyperdistensibility of the venous wall in this class of patients. The study also refers to ultrasound findings of reduced calf muscle pump and increased venous compliance.22 Lugli et al23 showed differences in flow when comparing C0a and C0s individuals using a highly sensitive flat probe continuous wave Doppler, finding the presence of bidirectional flow in symptomatic individuals and only unidirectional flow in the control group.
These changes in the microcirculation (Figure 1 and Table I) associated with genetic and environmental factors such as sedentary lifestyle, type of occupation, and personal habits may be at the origin of symptoms and the onset of an insidious chronic inflammatory process in C0 and C1 patients. Evidence shows the role of several inflammatory mediators released in the activation of type C nocireceptors, located in the middle layer of the venous wall and in the perivenous connective tissue, as responsible for the symptoms in these patients.5,24
C2 and C3 patients
Patients considered C2 class have varicose veins with at least 3 mm in diameter and no limb edema. The clinical picture can vary considerably, from completely asymptomatic individuals to complaints of constant heaviness and pain associated with standing. At the level of microcirculation, changes in the diameter of the dermal papilla and the growth of the capillary bulk are already evident. Thirty percent of capillaries present morphological changes due to vascular remodeling, but functional capillary density has normal values. Microcirculatory parameters gradually change. As the disease progresses to C3 class, when edema appears on physical examination, around half of the capillaries have already undergone remodeling.5
C3 class seems to be the point of no return for microcirculatory changes in CVD. Although there is a large overlap in values between CEAP classes, it is possible to observe changes in microcirculatory parameters at this stage of the disease, especially the number of active capillaries that begin to reduce, albeit in a soft way, and a large contingent of remodeled cutaneous ones, when about half of the normal hairpin-shaped vessels were replaced by glomerulus-like capillaries (Figure 1 and Table I).
C4, C5, and C6 patients
In advanced stages of CVD, especially those recognized as chronic venous insufficiency equivalent to CEAP clinical classes C4, C5, and C6, clinical signs and symptoms tend to be more exuberant. There may be evident skin pigmentation, and healthy skin gives way to fibrotic tissue that compromises the dermis and subcutaneous cellular tissue and the lymphatic system.
Capillary remodeling and changes in vascular permeability are associated with elevated levels of vascular endothelial growth factor in patients at these stages of CVD. The development of skin fibrosis may be caused by high levels of transforming growth factor β type 1 (TGF-β1) present in the skin of these patients. TGF-β1 is produced by activated leukocytes, and it stimulates the production of excess fibrinogen and collagen, causing subcutaneous fibrosis.18
In addition to skin fibrosis, it is also from C4 stage of CVD that skin pigmentation occurs. Cutaneous deposits of hemosiderin are caused by extravasation of red blood cells, which in turn release hemoglobin and iron into the interstitial space, increasing the activity of MMPs and causing tissue damage.
In the cutaneous capillaries, the predominant morphology is large vascular coils, reaching 75% of the capillaries in C5 patients. The remodeling also involves the dermal papillae, which doubles in size when comparing C1 and C5 patients (Figure 1 and Table I).5 There is already a significant reduction in functional capillary density. In C4 and C5, only 12 capillaries/mm2 are counted, approximately half of the active functional capillary units compared with early stages of CVD. In more advanced disease stages, therefore, we find a scarce cutaneous capillary network with vessels of completely anomalous morphology. It is possible to identify a vascularized areas that correspond to lesions known as atrophie blanche sign found on clinical examination.25
Classes C4, C5, and C6 represent the terminal stage of CVD, presenting irreversible sequelae in most cases, a consequence of chronic inflammatory effects over years of disease acting on the venous network of the macrocirculation and microcirculation, and even after successful interventions. Successful treatments at this stage of the disease are not capable of restoring the physiological status of these patients.
Conclusion
The progression of CVD is associated with the perpetuation of an inflammatory process that translates early into microscopic changes at the level of the venous wall and valve structures, and the extensive cutaneous capillary network of the lower limbs. Over the last few decades, knowledge of the microcirculation has expanded its pathophysiological and molecular bases and, above all, its correlations with clinical practice. As this understanding advances, new insights and possibilities emerge for research in this territory that represents the interface between biomolecular changes and clinical practice in the care of patients with CVD.

CORRESPONDING AUTHOR
Eliete BOUSKELA, MD, PhD
Laboratório de Pesquisas Clínicas e
Experimentais em Biologia Vascular
– BioVasc; Pavilhão Reitor Haroldo
Lisboa da Cunha, térreo; Universidade
do Estado do Rio de Janeiro; Rua São
Francisco Xavier, 524;20550-013 Rio de
Janeiro RJ, Brazil
email: eliete.bouskela@gmail.com
References
1. Fagrell B. Structural changes of human capillaries in chronic arterial and venous insufficiency. Bibl Anat. 1981;20:645-648.
2. Khilnani NM, Davies AH. CEAP: a review of the 2020 revision. Phlebology. 2020;35(10):745-748.
3. Lurie F, Passman M, Meisner M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8(3):342-352.
4. Venous hemodynamic changes in lower limb venous disease: the UIP consensus according to scientific evidence. Int Angiol. 2016;35(3):236-352.
5. Virgini-Magalhães CE, Porto CL, Fernandes FF, Dorigo DM, Bottino DA, Bouskela E. Use of microcirculatory parameters to evaluate chronic venous insufficiency. J Vasc Surg. 2006;43:1037-1044.
6. Lascasas-Porto CL, Milhomens LM, Virgini Magalhães CE, Fernandes FF, Sicuro FL, Bouskela E. Use of microcirculatory parameters to evaluate clinical treatments of chronic venous disorder (CVD). Microvasc Res. 2008;76:66-72.
7. Virgini-Magalhães CE, Porto CLL, Fernandes FFA, Dorigo DM, Bottino DA, Bouskela E. Quantification of microangiopathy in chronic venous disease. Phlebolymphology. 2007;14:129-134.
8. Fagrell B, Intaglietta M. Microcirculation: its significance in clinical and molecular medicine. J Intern Med. 1997;241(5):349- 362.
9. Mouton WG, Habegger AK, Haenni B, Tschanz S, Baumgartner I, Ochs M. Valve disease in chronic venous disorders: a quantitative ultrastructural analysis by transmission electron microscopy and stereology. Swiss Med Wkly. 2013;143:w13755.
10. Vincent JR, Jones GT, Hill GB, van Rij AM. Failure of microvenous valves in small superficial veins is a key to the skin changes of venous insufficiency. J Vasc Surg. 2011;54:62S-69S.
11. Govind D, Thomas KN, Hill BG, Van Rij AM. Microvenous reflux in the skin of limbs with superficial venous incompetence. Ultrasound Med Biol. 2018;44(4):756- 761.
12. Costa D, Andreucci M, Ielapi N, et al. Molecular determinants of chronic venous disease: a comprehensive review. Int J Mol Sci. 2023;24:1928.
13. Theofilis P, Sagris M, Oikonomou E, et al. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines. 2021;9(7):781.
14. Ortega MA, Fraile-Martínez O, García Montero C, et al. Understanding chronic venous disease: a critical overview of its pathophysiology and medical management. J Clin Med. 2021;10:3239.
15. Gwozdzinski L, Pieniazek A, Gwozdzinski K. Factors influencing venous remodeling in the development of varicose veins of the lower limbs. Int J Mol Sci. 2024;25:1560.
16. Howlader MH, Smith PDC. Correlation of severity of chronic venous disease with capillary morphology assessed by capillary microscopy. J Vasc Surg. 2006;43(3):563- 569.
17. Wang X, Khalil RA. Matrix metalloproteinases, vascular remodeling, and vascular disease. Adv Pharmacol. 2018;81:241-330.
18. Mansilha A, Sousa J. Pathophysiological mechanisms of chronic venous disease and implications for venoactive drug therapy. Int J Mol Sci. 2018;19:1669.
19. Serra R, Andreucci M, De Caridi G, Massara M, Mastroroberto P, de Franciscis S. Functional chronic venous disease: a systematic review. Phlebology. 2017;32(9):588-592.
20. Tsoukanov YT, Tsoukanov AY, Nikolaychuk A. Great saphenous vein transitory reflux in patients with symptoms related to chronic venous disorders, but without visible signs (C0s), and its correction with MPFF treatment. Phlebolymphology. 2015;22(1):25.
21. Senra Barros B, Kakkos SK, De Maeseneer M, Nicolaides AN. Chronic venous disease: from symptoms to microcirculation. Int Angiol. 2019;38(3):211-218.
22. Andreozzi GM, Signorelli S, Di Pino L, et al. Varicose symptoms without varicose veins: the hypotonic phlebopathy, epidemiology and pathophysiology. The Acireale project. Minerva Cardioangiol. 2000;48(10):277- 285.
23. Lugli M, Maleti O, Iabichella ML, Perrin M. Investigation of non-saphenous veins in C0S patients. Int Angiol. 2018;37(2):169- 175.
24. Guex JJ, Rabe E, Escotto SI, et al. The “C0s” patient: worldwide results from the Vein Consult Program. Phlebolymphology. 2012;19(4):182-192.
25. Nicolaides A, Kakkos S, Baekgaard N, et al. Management of chronic venous disorders of the lower limbs. Guidelines According to Scientific Evidence. Part I. Int Angiol. 2018;37(3):181-254
